Analysis of adherence, biofilm formation and cytotoxicity suggests a greater virulence potential of Gardnerella vaginalis relative to other bacterial-vaginosis-associated anaerobes
- PMID: 19910411
- PMCID: PMC2890091
- DOI: 10.1099/mic.0.034280-0
Analysis of adherence, biofilm formation and cytotoxicity suggests a greater virulence potential of Gardnerella vaginalis relative to other bacterial-vaginosis-associated anaerobes
Abstract
Worldwide, bacterial vaginosis (BV) is the most common vaginal disorder in women of childbearing age. BV is characterized by a dramatic shift in the vaginal microflora, involving a relative decrease in lactobacilli, and a proliferation of anaerobes. In most cases of BV, the predominant bacterial species found is Gardnerella vaginalis. However, pure cultures of G. vaginalis do not always result in BV, and asymptomatic women are sometimes colonized with low numbers of G. vaginalis. Thus, there is controversy about whether G. vaginalis is an opportunistic pathogen and the causative agent of many cases of BV, or whether BV is a polymicrobial condition caused by the collective effects of an altered microbial flora. Recent studies of the biofilm-forming potential and cytotoxic activity of G. vaginalis have renewed interest in the virulence potential of this organism. In an effort to tease apart the aetiology of this disorder, we utilized in vitro assays to compare three virulence properties of G. vaginalis relative to other BV-associated anaerobes. We designed a viable assay to analyse bacterial adherence to vaginal epithelial cells, we compared biofilm-producing capacities, and we assessed cytotoxic activity. Of the BV-associated anaerobes tested, only G. vaginalis demonstrated all three virulence properties combined. This study suggests that G. vaginalis is more virulent than other BV-associated anaerobes, and that many of the bacterial species frequently isolated from BV may be relatively avirulent opportunists that colonize the vagina after G. vaginalis has initiated an infection.
Figures

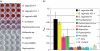

References
-
- Biagi, E., Vitali, B., Pugliese, C., Candela, M., Donders, G. G. & Brigidi, P. (2009). Quantitative variations in the vaginal bacterial population associated with asymptomatic infections: a real-time polymerase chain reaction study. Eur J Clin Microbiol Infect Dis 28, 281–285. - PubMed
-
- Cauci, S., Driussi, S., Guaschino, S., Isola, M. & Quadrifoglio, F. (2002). Correlation of local interleukin-1β levels with specific IgA response against Gardnerella vaginalis cytolysin in women with bacterial vaginosis. Am J Reprod Immunol 47, 257–264. - PubMed
-
- Cauci, S., Culhane, J.F., Di Santolo, M. & McCollum, K. (2008). Among pregnant women with bacterial vaginosis, the hydrolytic enzymes sialidase and prolidase are positively associated with interleukin-1beta. Am J Obstet Gynecol 198, 132.e1–7. - PubMed
-
- Criswell, B. S., Ladwig, C. L., Gardner, H. L. & Dukes, C. D. (1969). Haemophilus vaginalis: vaginitis by inoculation from culture. Obstet Gynecol 33, 195–199. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

