Role of host cell polarity and leading edge properties in Pseudomonas type III secretion
- PMID: 19910414
- PMCID: PMC2890084
- DOI: 10.1099/mic.0.033241-0
Role of host cell polarity and leading edge properties in Pseudomonas type III secretion
Abstract
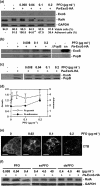
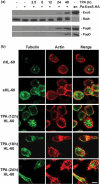
Type III secretion (T3S) functions in establishing infections in a large number of Gram-negative bacteria, yet little is known about how host cell properties might function in this process. We used the opportunistic pathogen Pseudomonas aeruginosa and the ability to alter host cell sensitivity to Pseudomonas T3S to explore this problem. HT-29 epithelial cells were used to study cellular changes associated with loss of T3S sensitivity, which could be induced by treatment with methyl-beta-cyclodextrin or perfringolysin O. HL-60 promyelocytic cells are innately resistant to Pseudomonas T3S and were used to study cellular changes occurring in response to induction of T3S sensitivity, which occurred following treatment with phorbol esters. Using both cell models, a positive correlation was observed between eukaryotic cell adherence to tissue culture wells and T3S sensitivity. In examining the type of adhesion process linked to T3S sensitivity in HT-29 cells, a hierarchical order of protein involvement was identified that paralleled the architecture of leading edge (LE) focal complexes. Conversely, in HL-60 cells, induction of T3S sensitivity coincided with the onset of LE properties and the development of actin-rich projections associated with polarized cell migration. When LE architecture was examined by immunofluorescent staining for actin, Rac1, IQ-motif-containing GTPase-activating protein 1 (IQGAP1) and phosphatidylinositol 3 kinase (PI3 kinase), intact LE structure was found to closely correlate with host cell sensitivity to P. aeruginosa T3S. Our model for host cell involvement in Pseudomonas T3S proposes that cortical actin polymerization at the LE alters membrane properties to favour T3S translocon function and the establishment of infections, which is consistent with Pseudomonas infections targeting wounded epithelial barriers undergoing cell migration.
Figures





Similar articles
-
Examining the role of actin-plasma membrane association in Pseudomonas aeruginosa infection and type III secretion translocation in migratory T24 epithelial cells.Infect Immun. 2012 Sep;80(9):3049-64. doi: 10.1128/IAI.00231-12. Epub 2012 Jun 11. Infect Immun. 2012. PMID: 22689823 Free PMC article.
-
Injection of Pseudomonas aeruginosa Exo toxins into host cells can be modulated by host factors at the level of translocon assembly and/or activity.PLoS One. 2012;7(1):e30488. doi: 10.1371/journal.pone.0030488. Epub 2012 Jan 27. PLoS One. 2012. PMID: 22299042 Free PMC article.
-
Characterization of an ExoS Type III translocation-resistant cell line.Infect Immun. 2005 Jan;73(1):638-43. doi: 10.1128/IAI.73.1.638-643.2005. Infect Immun. 2005. PMID: 15618208 Free PMC article.
-
Protein export according to schedule: architecture, assembly, and regulation of type III secretion systems from plant- and animal-pathogenic bacteria.Microbiol Mol Biol Rev. 2012 Jun;76(2):262-310. doi: 10.1128/MMBR.05017-11. Microbiol Mol Biol Rev. 2012. PMID: 22688814 Free PMC article. Review.
-
The bacterial injection kit: type III secretion systems.Ann Med. 2005;37(4):234-49. doi: 10.1080/07853890510037329. Ann Med. 2005. PMID: 16019722 Review.
Cited by
-
Gene expression profile analysis of ventilator-associated pneumonia.Mol Med Rep. 2015 Nov;12(5):7455-62. doi: 10.3892/mmr.2015.4389. Epub 2015 Sep 29. Mol Med Rep. 2015. PMID: 26459786 Free PMC article.
-
The Pseudomonas aeruginosa N-acylhomoserine lactone quorum sensing molecules target IQGAP1 and modulate epithelial cell migration.PLoS Pathog. 2012;8(10):e1002953. doi: 10.1371/journal.ppat.1002953. Epub 2012 Oct 11. PLoS Pathog. 2012. PMID: 23071436 Free PMC article.
-
Metastatic MTLn3 and non-metastatic MTC adenocarcinoma cells can be differentiated by Pseudomonas aeruginosa.Biol Open. 2013 Jul 3;2(9):891-900. doi: 10.1242/bio.20133632. eCollection 2013. Biol Open. 2013. PMID: 24143275 Free PMC article.
-
The biology of IQGAP proteins: beyond the cytoskeleton.EMBO Rep. 2015 Apr;16(4):427-46. doi: 10.15252/embr.201439834. Epub 2015 Feb 26. EMBO Rep. 2015. PMID: 25722290 Free PMC article. Review.
-
Modified needle-tip PcrV proteins reveal distinct phenotypes relevant to the control of type III secretion and intoxication by Pseudomonas aeruginosa.PLoS One. 2011 Mar 29;6(3):e18356. doi: 10.1371/journal.pone.0018356. PLoS One. 2011. PMID: 21479247 Free PMC article.
References
-
- Barber, D. F., Bartolomé, A., Hernandez, C., Flores, J. M., Redondo, C., Fernandez-Arias, C., Camps, M., Rückle, T., Schwarz, M. K. & other authors (2005). PI3Kγ inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med 11, 933–935. - PubMed
-
- Camps, M., Rückle, T., Ji, H., Ardissone, V., Rintelen, F., Shaw, J., Ferrandi, C., Chabert, C., Gillieron, C. & other authors (2005). Blockade of PI3K suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med 11, 936–943. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

