Jmjd1a demethylase-regulated histone modification is essential for cAMP-response element modulator-regulated gene expression and spermatogenesis
- PMID: 19910458
- PMCID: PMC2807331
- DOI: 10.1074/jbc.M109.066845
Jmjd1a demethylase-regulated histone modification is essential for cAMP-response element modulator-regulated gene expression and spermatogenesis
Abstract
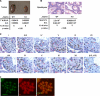
Spermatogenesis, a fundamental process in the male reproductive system, requires a series of tightly controlled epigenetic and genetic events in germ cells ranging from spermatogonia to spermatozoa. Jmjd1a is a key epigenetic regulator expressed in the testis. It specifically demethylates mono- and di-methylated histone H3 lysine 9 (H3K9me1 and H3K9me2) but not tri-methylated H3K9 (H3K9me3). In this study, we generated a Jmjd1a antibody for immunohistochemistry and found Jmjd1a was specifically produced in pachytene and secondary spermatocytes. Disruption of the Jmjd1a gene in mice significantly increased H3K9me1 and H3K9me2 levels in pachytene spermatocytes and early elongating spermatids without affecting H3K9me3 levels. Concurrently, the levels of histone acetylation were decreased in Jmjd1a knock-out germ cells. This suggests Jmjd1a promotes transcriptional activation by lowering histone methylation and increasing histone acetylation. Interestingly, the altered histone modifications in Jmjd1a-deficient germ cells caused diminished cAMP-response element modulator (Crem) recruitment to chromatin and decreased expression of the Crem coactivator Act and their target genes Tnp1 (transition protein 1), Tnp2, Prm1 (protamine 1), and Prm2, all of which are essential for chromatin condensation in spermatids. In agreement with these findings, Jmjd1a deficiency caused extensive germ cell apoptosis and blocked spermatid elongation, resulting in severe oligozoospermia, small testes, and infertility in male mice. These results indicate that the Jmjd1a-controlled epigenetic histone modifications are crucial for Crem-regulated gene expression and spermatogenesis.
Figures



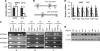



Similar articles
-
Deficient expression of JMJD1A histone demethylase in patients with round spermatid maturation arrest.Reprod Biomed Online. 2017 Jan;34(1):82-89. doi: 10.1016/j.rbmo.2016.09.005. Epub 2016 Sep 22. Reprod Biomed Online. 2017. PMID: 27692601
-
The testis-enriched histone demethylase, KDM4D, regulates methylation of histone H3 lysine 9 during spermatogenesis in the mouse but is dispensable for fertility.Biol Reprod. 2011 Jun;84(6):1225-34. doi: 10.1095/biolreprod.110.088955. Epub 2011 Feb 3. Biol Reprod. 2011. PMID: 21293030 Free PMC article.
-
Round spermatids show normal testis-specific H1t but reduced cAMP-responsive element modulator and transition protein 1 expression in men with round-spermatid maturation arrest.J Androl. 1999 Nov-Dec;20(6):747-54. J Androl. 1999. PMID: 10591614
-
Histone demethylase JHDM2A is involved in male infertility and obesity.J Androl. 2010 Jan-Feb;31(1):75-8. doi: 10.2164/jandrol.109.008052. Epub 2009 Oct 29. J Androl. 2010. PMID: 19875498 Free PMC article. Review.
-
Regulation of gene expression in post-meiotic male germ cells: CREM-signalling pathways and male fertility.Hum Fertil (Camb). 2006 Jun;9(2):73-9. doi: 10.1080/14647270500463400. Hum Fertil (Camb). 2006. PMID: 16825108 Review.
Cited by
-
Kdm3a lysine demethylase is an Hsp90 client required for cytoskeletal rearrangements during spermatogenesis.Mol Biol Cell. 2014 Apr;25(8):1216-33. doi: 10.1091/mbc.E13-08-0471. Epub 2014 Feb 19. Mol Biol Cell. 2014. PMID: 24554764 Free PMC article.
-
Potential Role of CREM in Diabetes-Associated Testicular Dysfunction: Current Evidence and Future Perspectives.Reprod Med Biol. 2025 Jun 5;24(1):e12661. doi: 10.1002/rmb2.12661. eCollection 2025 Jan-Dec. Reprod Med Biol. 2025. PMID: 40474948 Free PMC article. Review.
-
Expression Analysis of Circular RNAs in Young and Sexually Mature Boar Testes.Animals (Basel). 2021 May 17;11(5):1430. doi: 10.3390/ani11051430. Animals (Basel). 2021. PMID: 34067577 Free PMC article.
-
Combined Loss of JMJD1A and JMJD1B Reveals Critical Roles for H3K9 Demethylation in the Maintenance of Embryonic Stem Cells and Early Embryogenesis.Stem Cell Reports. 2018 Apr 10;10(4):1340-1354. doi: 10.1016/j.stemcr.2018.02.002. Epub 2018 Mar 8. Stem Cell Reports. 2018. PMID: 29526734 Free PMC article.
-
Posttranscriptional regulation of gene expression by Piwi proteins and piRNAs.Mol Cell. 2014 Oct 2;56(1):18-27. doi: 10.1016/j.molcel.2014.09.012. Mol Cell. 2014. PMID: 25280102 Free PMC article. Review.
References
-
- Russell L. D., Ettlin R. A., Sinha-Hikim A. P., Clegg E. D. (1990) Histological and Histopathological Evaluation of the Testis, pp. 1–161, Cache River Press, Clearwater, FL
-
- Mali P., Kaipia A., Kangasniemi M., Toppari J., Sandberg M., Hecht N. B., Parvinen M. (1989) Reprod. Fertil. Dev. 1, 369–382 - PubMed
-
- Nantel F., Monaco L., Foulkes N. S., Masquilier D., LeMeur M., Henriksén K., Dierich A., Parvinen M., Sassone-Corsi P. (1996) Nature 380, 159–162 - PubMed
-
- Cho C., Willis W. D., Goulding E. H., Jung-Ha H., Choi Y. C., Hecht N. B., Eddy E. M. (2001) Nat. Genet. 28, 82–86 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

