Ethanol enhances hepatitis C virus replication through lipid metabolism and elevated NADH/NAD+
- PMID: 19910460
- PMCID: PMC2801286
- DOI: 10.1074/jbc.M109.045740
Ethanol enhances hepatitis C virus replication through lipid metabolism and elevated NADH/NAD+
Abstract
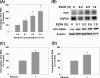
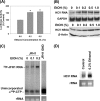
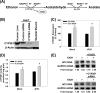
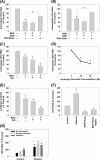
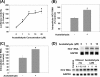
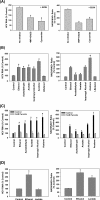
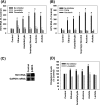
Ethanol has been suggested to elevate HCV titer in patients and to increase HCV RNA in replicon cells, suggesting that HCV replication is increased in the presence and absence of the complete viral replication cycle, but the mechanisms remain unclear. In this study, we use Huh7 human hepatoma cells that naturally express comparable levels of CYP2E1 as human liver to demonstrate that ethanol, at subtoxic and physiologically relevant concentrations, enhances complete HCV replication. The viral RNA genome replication is affected for both genotypes 2a and 1b. Acetaldehyde, a major product of ethanol metabolism, likewise enhances HCV replication at physiological concentrations. The potentiation of HCV replication by ethanol is suppressed by inhibiting CYP2E1 or aldehyde dehydrogenase and requires an elevated NADH/NAD(+) ratio. In addition, acetate, isopropyl alcohol, and concentrations of acetone that occur in diabetics enhance HCV replication with corresponding increases in the NADH/NAD(+). Furthermore, inhibiting the host mevalonate pathway with lovastatin or fluvastatin and fatty acid synthesis with 5-(tetradecyloxy)-2-furoic acid or cerulenin significantly attenuates the enhancement of HCV replication by ethanol, acetaldehyde, acetone, as well as acetate, whereas inhibiting beta-oxidation with beta-mercaptopropionic acid increases HCV replication. Ethanol, acetaldehyde, acetone, and acetate increase the total intracellular cholesterol content, which is attenuated with lovastatin. In contrast, both endogenous and exogenous ROS suppress the replication of HCV genotype 2a, as previously shown with genotype 1b.
Conclusion: Therefore, lipid metabolism and alteration of cellular NADH/NAD(+) ratio are likely to play a critical role in the potentiation of HCV replication by ethanol rather than oxidative stress.
Figures
Similar articles
-
The influence of cholesterol and lipid metabolism on host cell structure and hepatitis C virus replication.Biochem Cell Biol. 2006 Feb;84(1):67-79. doi: 10.1139/o05-149. Biochem Cell Biol. 2006. PMID: 16462891
-
Mitochondrial oxidative stress and CD95 ligand: a dual mechanism for hepatocyte apoptosis in chronic alcoholism.Hepatology. 2002 May;35(5):1205-14. doi: 10.1053/jhep.2002.32969. Hepatology. 2002. PMID: 11981771
-
Alcohol metabolism increases the replication of hepatitis C virus and attenuates the antiviral action of interferon.J Infect Dis. 2008 Dec 15;198(12):1766-75. doi: 10.1086/593216. J Infect Dis. 2008. PMID: 18956976
-
Reliance of host cholesterol metabolic pathways for the life cycle of hepatitis C virus.PLoS Pathog. 2007 Aug 31;3(8):e108. doi: 10.1371/journal.ppat.0030108. PLoS Pathog. 2007. PMID: 17784784 Free PMC article. Review.
-
Impact of alcohol on hepatitis C virus replication and interferon signaling.World J Gastroenterol. 2010 Mar 21;16(11):1337-43. doi: 10.3748/wjg.v16.i11.1337. World J Gastroenterol. 2010. PMID: 20238400 Free PMC article. Review.
Cited by
-
Predictors of alcohol use among rural drug users after disclosure of hepatitis C virus status.J Stud Alcohol Drugs. 2013 May;74(3):386-95. doi: 10.15288/jsad.2013.74.386. J Stud Alcohol Drugs. 2013. PMID: 23490567 Free PMC article.
-
Viral antibody response predicts morbidity and mortality in alcohol-associated hepatitis.Hepatology. 2025 Jul 1;82(1):127-139. doi: 10.1097/HEP.0000000000001046. Epub 2024 Sep 25. Hepatology. 2025. PMID: 39325984
-
Ethanol facilitates hepatitis C virus replication via up-regulation of GW182 and heat shock protein 90 in human hepatoma cells.Hepatology. 2013 Jan;57(1):70-80. doi: 10.1002/hep.26010. Hepatology. 2013. PMID: 22898980 Free PMC article.
-
NADH elevation during chronic hypoxia leads to VHL-mediated HIF-1α degradation via SIRT1 inhibition.Cell Biosci. 2023 Sep 30;13(1):182. doi: 10.1186/s13578-023-01130-3. Cell Biosci. 2023. PMID: 37777750 Free PMC article.
-
Predictors and effects of alcohol use on liver function among young HCV-infected injection drug users in a behavioral intervention.J Hepatol. 2011 Jul;55(1):45-52. doi: 10.1016/j.jhep.2010.10.028. Epub 2010 Nov 24. J Hepatol. 2011. PMID: 21145862 Free PMC article. Clinical Trial.
References
-
- Jamal M. M., Morgan T. R. (2003) Best Pract. Res. Clin. Gastroenterol. 17, 649–662 - PubMed
-
- Sata M., Fukuizumi K., Uchimura Y., Nakano H., Ishii K., Kumashiro R., Mizokami M., Lau J. Y., Tanikawa K. (1996) J. Viral. Hepat. 3, 143–148 - PubMed
-
- Zhang T., Li Y., Lai J. P., Douglas S. D., Metzger D. S., O'Brien C. P., Ho W. Z. (2003) Hepatology 38, 57–65 - PubMed
-
- Seronello S., Sheikh M. Y., Choi J. (2007) Free Radic. Biol. Med. 43, 869–882 - PubMed
-
- Cromie S. L., Jenkins P. J., Bowden D. S., Dudley F. J. (1996) J. Hepatol. 25, 821–826 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

