Mobility, microtubule nucleation and structure of microtubule-organizing centers in multinucleated hyphae of Ashbya gossypii
- PMID: 19910487
- PMCID: PMC2801712
- DOI: 10.1091/mbc.e09-01-0063
Mobility, microtubule nucleation and structure of microtubule-organizing centers in multinucleated hyphae of Ashbya gossypii
Abstract
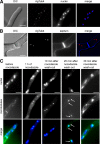
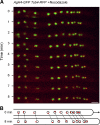
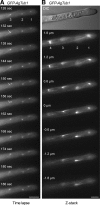
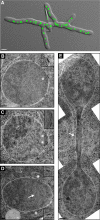
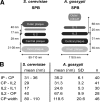
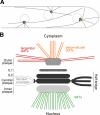
We investigated the migration of multiple nuclei in hyphae of the filamentous fungus Ashbya gossypii. Three types of cytoplasmic microtubule (cMT)-dependent nuclear movements were characterized using live cell imaging: short-range oscillations (up to 4.5 microm/min), rotations (up to 180 degrees in 30 s), and long-range nuclear bypassing (up to 9 microm/min). These movements were superimposed on a cMT-independent mode of nuclear migration, cotransport with the cytoplasmic stream. This latter mode is sufficient to support wild-type-like hyphal growth speeds. cMT-dependent nuclear movements were led by a nuclear-associated microtubule-organizing center, the spindle pole body (SPB), which is the sole site of microtubule nucleation in A. gossypii. Analysis of A. gossypii SPBs by electron microscopy revealed an overall laminar structure similar to the budding yeast SPB but with distinct differences at the cytoplasmic side. Up to six perpendicular and tangential cMTs emanated from a more spherical outer plaque. The perpendicular and tangential cMTs most likely correspond to short, often cortex-associated cMTs and to long, hyphal growth-axis-oriented cMTs, respectively, seen by in vivo imaging. Each SPB nucleates its own array of cMTs, and the lack of overlapping cMT arrays between neighboring nuclei explains the autonomous nuclear oscillations and bypassing observed in A. gossypii hyphae.
Figures



References
-
- Alberti-Segui C., Dietrich F., Altmann-Johl R., Hoepfner D., Philippsen P. Cytoplasmic dynein is required to oppose the force that moves nuclei towards the hyphal tip in the filamentous ascomycete Ashbya gossypii. J. Cell Sci. 2001;114:975–986. - PubMed
-
- Ayad-Durieux Y., Knechtle P., Goff S., Dietrich F., Philippsen P. A PAK-like protein kinase is required for maturation of young hyphae and septation in the filamentous ascomycete Ashbya gossypii. J. Cell Sci. 2000;113:4563–4575. - PubMed
-
- Bullitt E., Rout M. P., Kilmartin J. V., Akey C. W. The yeast spindle pole body is assembled around a central crystal of Spc42p. Cell. 1997;89:1077–1086. - PubMed
-
- Byers B., Goetsch L. Duplication of spindle plaques and integration of the yeast cell cycle. Cold Spring Harb. Symp. Quant. Biol. 1974;38:123–131. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

