Mechanisms of self-organization of cortical microtubules in plants revealed by computational simulations
- PMID: 19910489
- PMCID: PMC2808237
- DOI: 10.1091/mbc.e09-07-0579
Mechanisms of self-organization of cortical microtubules in plants revealed by computational simulations
Abstract
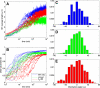
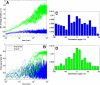
Microtubules confined to the two-dimensional cortex of elongating plant cells must form a parallel yet dispersed array transverse to the elongation axis for proper cell wall expansion. Some of these microtubules exhibit free minus-ends, leading to migration at the cortex by hybrid treadmilling. Collisions between microtubules can result in plus-end entrainment ("zippering") or rapid depolymerization. Here, we present a computational model of cortical microtubule organization. We find that plus-end entrainment leads to self-organization of microtubules into parallel arrays, whereas catastrophe-inducing collisions do not. Catastrophe-inducing boundaries (e.g., upper and lower cross-walls) can tune the orientation of an ordered array to a direction transverse to elongation. We also find that changes in dynamic instability parameters, such as in mor1-1 mutants, can impede self-organization, in agreement with experimental data. Increased entrainment, as seen in clasp-1 mutants, conserves self-organization, but delays its onset and fails to demonstrate increased ordering. We find that branched nucleation at acute angles off existing microtubules results in distinctive sparse arrays and infer either that microtubule-independent or coparallel nucleation must dominate. Our simulations lead to several testable predictions, including the effects of reduced microtubule severing in katanin mutants.
Figures



References
-
- Baulin V. A., Marques C. M., Thalmann F. Collision induced spatial organization of microtubules. Biophys. Chem. 2007;128:231–244. - PubMed
-
- Burbank K. S., Mitchison T. J., Fisher D. S. Slide-and-cluster models for spindle assembly. Curr. Biol. 2007;17:1373–1383. - PubMed
-
- Chan J., Calder G., Fox S., Lloyd C. Cortical microtubule arrays undergo rotary movements in Arabidopsis hypocotyl epidermal cells. Nat. Cell Biol. 2007;9:171–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

