Farnesoid X receptor critically determines the fibrotic response in mice but is expressed to a low extent in human hepatic stellate cells and periductal myofibroblasts
- PMID: 19910507
- PMCID: PMC2789609
- DOI: 10.2353/ajpath.2009.090114
Farnesoid X receptor critically determines the fibrotic response in mice but is expressed to a low extent in human hepatic stellate cells and periductal myofibroblasts
Erratum in
- Am J Pathol. 2010 May;176(5):2581
Abstract
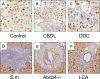
The nuclear bile acid receptor, farnesoid X receptor (FXR), may play a pivotal role in liver fibrosis. We tested the impact of genetic FXR ablation in four different mouse models. Hepatic fibrosis was induced in wild-type and FXR knock-out mice (FXR(-/-)) by CCl(4) intoxication, 3,5-diethoxycarbonyl-1,4-dihydrocollidine feeding, common bile duct ligation, or Schistosoma mansoni (S.m.)-infection. In addition, we determined nuclear receptor expression levels (FXR, pregnane X receptor (PXR), vitamin D receptor, constitutive androstane receptor (CAR), small heterodimer partner (SHP)) in mouse hepatic stellate cells (HSCs), portal myofibroblasts (MFBs), and human HSCs. Cell type-specific FXR protein expression was determined by immunohistochemistry in five mouse models and prototypic human fibrotic liver diseases. Expression of nuclear receptors was much lower in mouse and human HSCs/MFBs compared with total liver expression with the exception of vitamin D receptor. FXR protein was undetectable in mouse and human HSCs and MFBs. FXR loss had no effect in CCl(4)-intoxicated and S.m.-infected mice, but significantly decreased liver fibrosis of the biliary type (common bile duct ligation, 3,5-diethoxycarbonyl-1,4-dihydrocollidine). These data suggest that FXR loss significantly reduces fibrosis of the biliary type, but has no impact on non-cholestatic liver fibrosis. Since there is no FXR expression in HSCs and MFBs in liver fibrosis, our data indicate that these cells may not represent direct therapeutic targets for FXR ligands.
Figures







Comment in
-
New insights on the pathogenesis of biliary cirrhosis provided by studies in FXR knockout mice.J Hepatol. 2011 Oct;55(4):939-40. doi: 10.1016/j.jhep.2011.04.013. Epub 2011 May 11. J Hepatol. 2011. PMID: 21672564 Free PMC article.
References
-
- Zollner G, Marschall HU, Wagner M, Trauner M. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations. Mol Pharm. 2006;3:231–251. - PubMed
-
- Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y. FXR, a multipurpose nuclear receptor. Trends Biochem Sci. 2006;31:572–580. - PubMed
-
- Modica S, Moschetta A. Nuclear bile acid receptor FXR as pharmacological target: are we there yet? FEBS Lett. 2006;580:5492–5499. - PubMed
-
- Chen F, Ananthanarayanan M, Emre S, Neimark E, Bull LN, Knisely AS, Strautnieks SS, Thompson RJ, Magid MS, Gordon R, Balasubramanian N, Suchy FJ, Shneider BL. Progressive familial intrahepatic cholestasis, type 1, is associated with decreased farnesoid X receptor activity. Gastroenterology. 2004;126:756–764. - PubMed
-
- Kovacs P, Kress R, Rocha J, Kurtz U, Miquel JF, Nervi F, Mendez-Sanchez N, Uribe M, Bock HH, Schirin-Sokhan R, Stumvoll M, Mossner J, Lammert F, Wittenburg H. Variation of the gene encoding the nuclear bile salt receptor FXR and gallstone susceptibility in mice and humans. J Hepatol. 2008;48:116–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

