NADPH oxidases: functions and pathologies in the vasculature
- PMID: 19910640
- PMCID: PMC2841695
- DOI: 10.1161/ATVBAHA.108.181610
NADPH oxidases: functions and pathologies in the vasculature
Abstract
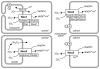
Reactive oxygen species are ubiquitous signaling molecules in biological systems. Four members of the NADPH oxidase (Nox) enzyme family are important sources of reactive oxygen species in the vasculature: Nox1, Nox2, Nox4, and Nox5. Signaling cascades triggered by stresses, hormones, vasoactive agents, and cytokines control the expression and activity of these enzymes and of their regulatory subunits, among which p22phox, p47phox, Noxa1, and p67phox are present in blood vessels. Vascular Nox enzymes are also regulated by Rac, ClC-3, Poldip2, and protein disulfide isomerase. Multiple Nox subtypes, simultaneously present in different subcellular compartments, produce specific amounts of superoxide, some of which is rapidly converted to hydrogen peroxide. The identity and location of these reactive oxygen species, and of the enzymes that degrade them, determine their downstream signaling pathways. Nox enzymes participate in a broad array of cellular functions, including differentiation, fibrosis, growth, proliferation, apoptosis, cytoskeletal regulation, migration, and contraction. They are involved in vascular pathologies such as hypertension, restenosis, inflammation, atherosclerosis, and diabetes. As our understanding of the regulation of these oxidases progresses, so will our ability to alter their functions and associated pathologies.
Figures

References
-
- Lassègue B, Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–297. - PubMed
-
- Zhang G, Zhang F, Muh R, Yi F, Chalupsky K, Cai H, Li PL. Autocrine/paracrine pattern of superoxide production through NAD(P)H oxidase in coronary arterial myocytes. Am J Physiol Heart Circ Physiol. 2007;292:H483–495. - PubMed
-
- Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. - PubMed
-
- Miller FJ, Jr, Filali M, Huss GJ, Stanic B, Chamseddine A, Barna TJ, Lamb FS. Cytokine activation of nuclear factor κ B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101:663–671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

