Inhibition of protein kinase CK2 closes the CFTR Cl channel, but has no effect on the cystic fibrosis mutant deltaF508-CFTR
- PMID: 19910675
- PMCID: PMC2795324
- DOI: 10.1159/000257427
Inhibition of protein kinase CK2 closes the CFTR Cl channel, but has no effect on the cystic fibrosis mutant deltaF508-CFTR
Abstract
Background: Deletion of phenylalanine-508 (DeltaF508) from the first nucleotide-binding domain (NBD1) in the wild-type cystic fibrosis (CF) transmembrane-conductance regulator (wtCFTR) causes CF. However, the mechanistic relationship between DeltaF508-CFTR and the diversity of CF disease is unexplained. The surface location of F508 on NBD1 creates the potential for protein-protein interactions and nearby, lies a consensus sequence (SYDE) reported to control the pleiotropic protein kinase CK2.
Methods: Electrophysiology, immunofluorescence and biochemistry applied to CFTR-expressing cells, Xenopus oocytes, pancreatic ducts and patient biopsies.
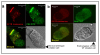
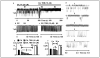
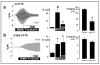
Results: Irrespective of PKA activation, CK2 inhibition (ducts, oocytes, cells) attenuates CFTR-dependent Cl(-) transport, closing wtCFTR in cell-attached membrane patches. CK2 and wtCFTR co-precipitate and CK2 co-localized with wtCFTR (but not DeltaF508-CFTR) in apical membranes of human airway biopsies. Comparing wild-type and DeltaF508CFTR expressing oocytes, only DeltaF508-CFTR Cl(-) currents were insensitive to two CK2 inhibitors. Furthermore, wtCFTR was inhibited by injecting a peptide mimicking the F508 region, whereas the DeltaF508-equivalent peptide had no effect.
Conclusions: CK2 controls wtCFTR, but not DeltaF508-CFTR. Others find that peptides from the F508 region of NBD1 allosterically control CK2, acting through F508. Hence, disruption of CK2-CFTR interaction by DeltaF508-CFTR might disrupt multiple, membrane-associated, CK2-dependent pathways, creating a new molecular disease paradigm for deleted F508 in CFTR.
2009 S. Karger AG, Basel.
Figures




Similar articles
-
Protein kinase CK2, cystic fibrosis transmembrane conductance regulator, and the deltaF508 mutation: F508 deletion disrupts a kinase-binding site.J Biol Chem. 2007 Apr 6;282(14):10804-13. doi: 10.1074/jbc.M610956200. Epub 2007 Feb 8. J Biol Chem. 2007. Retraction in: J Biol Chem. 2008 Sep 5;283(36):25103. PMID: 17289674 Retracted.
-
Modulation of protein kinase CK2 activity by fragments of CFTR encompassing F508 may reflect functional links with cystic fibrosis pathogenesis.Biochemistry. 2008 Jul 29;47(30):7925-36. doi: 10.1021/bi800316z. Epub 2008 Jul 3. Biochemistry. 2008. PMID: 18597485 Free PMC article.
-
Rattlesnake Phospholipase A2 Increases CFTR-Chloride Channel Current and Corrects ∆F508CFTR Dysfunction: Impact in Cystic Fibrosis.J Mol Biol. 2016 Jul 17;428(14):2898-915. doi: 10.1016/j.jmb.2016.05.016. Epub 2016 May 27. J Mol Biol. 2016. PMID: 27241308
-
Understanding protein kinase CK2 mis-regulation upon F508del CFTR expression.Naunyn Schmiedebergs Arch Pharmacol. 2011 Oct;384(4-5):473-88. doi: 10.1007/s00210-011-0650-x. Epub 2011 May 24. Naunyn Schmiedebergs Arch Pharmacol. 2011. PMID: 21607646 Free PMC article. Review.
-
Selective activation of cystic fibrosis transmembrane conductance regulator Cl- and HCO3- conductances.JOP. 2001 Jul;2(4 Suppl):212-8. JOP. 2001. PMID: 11875262 Review.
Cited by
-
Investigating CFTR and KCa3.1 Protein/Protein Interactions.PLoS One. 2016 Apr 19;11(4):e0153665. doi: 10.1371/journal.pone.0153665. eCollection 2016. PLoS One. 2016. PMID: 27092946 Free PMC article.
-
NM23 proteins: innocent bystanders or local energy boosters for CFTR?Lab Invest. 2018 Mar;98(3):272-282. doi: 10.1038/labinvest.2017.121. Epub 2017 Dec 18. Lab Invest. 2018. PMID: 29251738 Review.
-
CFTR induces extracellular acid sensing in Xenopus oocytes which activates endogenous Ca²⁺-activated Cl⁻ conductance.Pflugers Arch. 2011 Sep;462(3):479-87. doi: 10.1007/s00424-011-0983-9. Epub 2011 Jun 7. Pflugers Arch. 2011. PMID: 21647592
-
In silico search for modifier genes associated with pancreatic and liver disease in Cystic Fibrosis.PLoS One. 2017 Mar 24;12(3):e0173822. doi: 10.1371/journal.pone.0173822. eCollection 2017. PLoS One. 2017. PMID: 28339466 Free PMC article.
-
CK2 is a key regulator of SLC4A2-mediated Cl-/HCO3- exchange in human airway epithelia.Pflugers Arch. 2017 Sep;469(9):1073-1091. doi: 10.1007/s00424-017-1981-3. Epub 2017 Apr 28. Pflugers Arch. 2017. PMID: 28455748 Free PMC article.
References
-
- Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui L-C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. - PubMed
-
- Welsh MJ, Ramsey BW, Accurso F, Cutting GR. Cystic fibrosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Inherited Disease. McGraw-Hill Inc.; New York: 2001. pp. 5121–5188.
-
- Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79:S23–45. - PubMed
-
- Riordan JR. Assembly of functional CFTR chloride channels. Annu Rev Physiol. 2005;67:701–718. - PubMed
-
- Holland IB, Cole SPC, Kuchler K, Higgins CF. ABC Proteins: From Bacteria to Man. Academic Press; London: 2003.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
