AATF mediates an antiapoptotic effect of the unfolded protein response through transcriptional regulation of AKT1
- PMID: 19911006
- PMCID: PMC2854298
- DOI: 10.1038/cdd.2009.175
AATF mediates an antiapoptotic effect of the unfolded protein response through transcriptional regulation of AKT1
Abstract
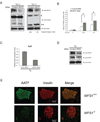
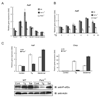
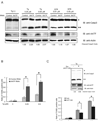
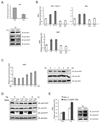
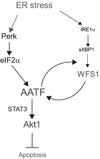
Endoplasmic reticulum (ER) stress-mediated cell death has an important role in the pathogenesis of chronic diseases, including diabetes and neurodegeneration. Although proapoptotic programs activated by ER stress have been extensively studied, identification and characterization of antiapoptotic programs that counteract ER stress are currently incomplete. Through the gene expression profiling of beta-cells lacking Wolfram syndrome 1 gene (WFS1), a causative gene for Wolfram syndrome, we discovered a novel antiapoptotic gene of the unfolded protein response (UPR), apoptosis antagonizing transcription factor (AATF). Here, we study the regulation of AATF, identify its target genes, and determine the basis for its antiapoptotic activities in response to ER stress. We show that AATF is induced by ER stress through the PERK-eIF2alpha pathway and transcriptionally activates the v-akt murine thymoma viral oncogene homolog 1 (AKT1) gene through signal transducer and activator of transcription 3 (Stat3), which sustains Akt1 activation and promotes cell survival. Ectopic expression of AATF or a constitutively active form of AKT1 confers on cells resistance to ER stress-mediated cell death, whereas RNAi-mediated knockdown of AATF or AKT1 renders cells sensitive to ER stress. We also discovered a positive crosstalk between the AATF and WFS1 signaling pathways. Thus, WFS1 deficiency or AATF deficiency mediates a self-perpetuating cycle of cell death. Our results reveal a novel antiapoptotic program relevant to the treatment of diseases caused by ER stress-mediated cell death.
Figures




References
-
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007 Jul;8(7):519–529. - PubMed
-
- Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci. 2007 Oct 4; - PubMed
-
- Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nature reviews. 2008 Dec;7(12):1013–1030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

