Simian varicella virus infection of rhesus macaques recapitulates essential features of varicella zoster virus infection in humans
- PMID: 19911054
- PMCID: PMC2770849
- DOI: 10.1371/journal.ppat.1000657
Simian varicella virus infection of rhesus macaques recapitulates essential features of varicella zoster virus infection in humans
Abstract
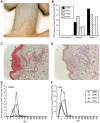
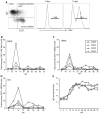
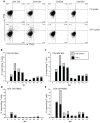
Simian varicella virus (SVV), the etiologic agent of naturally occurring varicella in primates, is genetically and antigenically closely related to human varicella zoster virus (VZV). Early attempts to develop a model of VZV pathogenesis and latency in nonhuman primates (NHP) resulted in persistent infection. More recent models successfully produced latency; however, only a minority of monkeys became viremic and seroconverted. Thus, previous NHP models were not ideally suited to analyze the immune response to SVV during acute infection and the transition to latency. Here, we show for the first time that intrabronchial inoculation of rhesus macaques with SVV closely mimics naturally occurring varicella (chickenpox) in humans. Infected monkeys developed varicella and viremia that resolved 21 days after infection. Months later, viral DNA was detected only in ganglia and not in non-ganglionic tissues. Like VZV latency in human ganglia, transcripts corresponding to SVV ORFs 21, 62, 63 and 66, but not ORF 40, were detected by RT-PCR. In addition, as described for VZV, SVV ORF 63 protein was detected in the cytoplasm of neurons in latently infected monkey ganglia by immunohistochemistry. We also present the first in depth analysis of the immune response to SVV. Infected animals produced a strong humoral and cell-mediated immune response to SVV, as assessed by immunohistology, serology and flow cytometry. Intrabronchial inoculation of rhesus macaques with SVV provides a novel model to analyze viral and immunological mechanisms of VZV latency and reactivation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Acute Simian Varicella Virus Infection Causes Robust and Sustained Changes in Gene Expression in the Sensory Ganglia.J Virol. 2016 Nov 14;90(23):10823-10843. doi: 10.1128/JVI.01272-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27681124 Free PMC article.
-
Abortive intrabronchial infection of rhesus macaques with varicella-zoster virus provides partial protection against simian varicella virus challenge.J Virol. 2015 Feb;89(3):1781-93. doi: 10.1128/JVI.03124-14. Epub 2014 Nov 19. J Virol. 2015. PMID: 25410871 Free PMC article.
-
Intrabronchial infection of rhesus macaques with simian varicella virus results in a robust immune response in the lungs.J Virol. 2014 Nov;88(21):12777-92. doi: 10.1128/JVI.01814-14. Epub 2014 Aug 20. J Virol. 2014. PMID: 25142604 Free PMC article.
-
Simian varicella: a model for human varicella-zoster virus infections.Rev Med Virol. 2004 Nov-Dec;14(6):363-81. doi: 10.1002/rmv.437. Rev Med Virol. 2004. PMID: 15386593 Review.
-
Varicella virus-mononuclear cell interaction.Adv Virus Res. 2003;62:1-17. doi: 10.1016/s0065-3527(03)62001-4. Adv Virus Res. 2003. PMID: 14719363 Review.
Cited by
-
Experimental infection of Cynomolgus Macaques (Macaca fascicularis) with human varicella-zoster virus.J Virol. 2012 Apr;86(7):3626-34. doi: 10.1128/JVI.06264-11. Epub 2012 Jan 18. J Virol. 2012. PMID: 22258257 Free PMC article.
-
Insights into the pathogenesis of varicella viruses.Curr Clin Microbiol Rep. 2019 Sep;6(3):156-165. doi: 10.1007/s40588-019-00119-2. Epub 2019 Jul 6. Curr Clin Microbiol Rep. 2019. PMID: 32999816 Free PMC article.
-
T-cell infiltration correlates with CXCL10 expression in ganglia of cynomolgus macaques with reactivated simian varicella virus.J Virol. 2013 Mar;87(5):2979-82. doi: 10.1128/JVI.03181-12. Epub 2012 Dec 26. J Virol. 2013. PMID: 23269790 Free PMC article.
-
Elevated serum substance P during simian varicella virus infection in rhesus macaques: implications for chronic inflammation and adverse cerebrovascular events.J Neurovirol. 2020 Dec;26(6):945-951. doi: 10.1007/s13365-020-00907-3. Epub 2020 Sep 22. J Neurovirol. 2020. PMID: 32964407 Free PMC article.
-
Pathogenesis of varicelloviruses in primates.J Pathol. 2015 Jan;235(2):298-311. doi: 10.1002/path.4451. J Pathol. 2015. PMID: 25255989 Free PMC article. Review.
References
-
- Wroblewska Z, Devlin M, Reilly K, van Trieste H, Wellish M, et al. The production of varicella Zoster virus antiserum in laboratory animals. Brief report. Arch Virol. 1982;74:233–238. - PubMed
-
- Felsenfeld AD, Schmidt NJ. Varicella-zoster virus immunizes patas monkeys against simian varicella-like disease. J Gen Virol. 1979;42:171–178. - PubMed
-
- Soike KF, Keller PM, Ellis RW. Immunization of monkeys with varicella-zoster virus glycoprotein antigens and their response to challenge with simian varicella virus. J Med Virol. 1987;22:307–313. - PubMed
-
- Myers MG, Connelly BL. Animal models of varicella. J Infect Dis. 1992;166(Suppl 1):S48–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5P51 RR00163-48/RR/NCRR NIH HHS/United States
- 5P51 RR00163-47/RR/NCRR NIH HHS/United States
- R01 AI066096/AI/NIAID NIH HHS/United States
- P01 AG032958/AG/NIA NIH HHS/United States
- AG032958/AG/NIA NIH HHS/United States
- 5P51 RR0163-45/RR/NCRR NIH HHS/United States
- K01 RR000163/RR/NCRR NIH HHS/United States
- R37 AG006127/AG/NIA NIH HHS/United States
- R01 AG006127/AG/NIA NIH HHS/United States
- P51 RR000163/RR/NCRR NIH HHS/United States
- P01 AG023664/AG/NIA NIH HHS/United States
- 5P51 RR00163/RR/NCRR NIH HHS/United States
- AG006127/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources

