Heme iron nitrosyl complex of MauG reveals an efficient redox equilibrium between hemes with only one heme exclusively binding exogenous ligands
- PMID: 19911786
- PMCID: PMC2801551
- DOI: 10.1021/bi9017544
Heme iron nitrosyl complex of MauG reveals an efficient redox equilibrium between hemes with only one heme exclusively binding exogenous ligands
Abstract
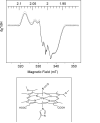
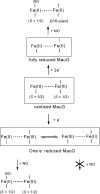
MauG is a diheme enzyme that oxidizes two protein-bound tryptophan residues to generate a catalytic tryptophan tryptophylquinone cofactor within methylamine dehydrogenase. Upon the two-electron oxidation of bis-ferric MauG, the two c-type hemes exist as a spin-uncoupled bis-Fe(IV) species with only one binding oxygen, which is chemically equivalent to a single ferryl heme plus a pi porphyrin cation radical ( Li , X. et al. ( 2008 ) Proc. Natl. Acad. Sci. U.S.A. 105 , 8597 - 8600 ). The EPR spectrum of the nitrosyl complex of fully reduced MauG shows a single six-coordinate Fe(II)-NO species, which is characteristic of a histidine-ligated Fe(II)-NO moiety in the heme environment. Exposure of partially reduced MauG to NO reveals a redox equilibrium with facile electron transfer between hemes but with only one binding nitric oxide. Thus, the second heme is able to stabilize all three redox states of iron (Fe(II), Fe(III), and Fe(IV)) in a six-coordinate protein-bound heme without binding exogenous ligands. This is unprecedented behavior for a protein-bound heme for which each of these redox states is relevant to the overall catalytic mechanism. The results also illustrate the electronic communication between the two iron centers, which function as a diheme unit rather than independent heme cofactors.
Figures

References
-
- Wang Y, Graichen ME, Liu A, Pearson AR, Wilmot CM, Davidson VL. MauG, a novel diheme protein required for tryptophan tryptophylquinone biogenesis. Biochemistry. 2003;42:7318–7325. - PubMed
-
- Davidson VL. Protein-derived cofactors. Expanding the scope of post-translational modifications. Biochemistry. 2007;46:5283–5292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

