Viremia, resuppression, and time to resistance in human immunodeficiency virus (HIV) subtype C during first-line antiretroviral therapy in South Africa
- PMID: 19911963
- PMCID: PMC2789416
- DOI: 10.1086/648444
Viremia, resuppression, and time to resistance in human immunodeficiency virus (HIV) subtype C during first-line antiretroviral therapy in South Africa
Abstract
Background: Episodes of viremia are common in African antiretroviral therapy (ART) programs. We sought to describe viremia, resuppression, and accumulation of resistance during first-line combination ART (cART) in South Africa.
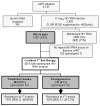
Methods: Retrospective analysis of a cohort receiving zidovudine, lamivudine, and either efavirenz or nevirapine with human immunodeficiency virus (HIV) RNA monitoring every 6 months. We assessed viremia (HIV RNA >1000 copies/mL after initial HIV RNA response) and resuppression (HIV RNA <400 copies/mL after viremia). Genotypic resistance testing was performed using stored plasma on a subset of patients at first detection of viremia and subsequently among patients with persistent viremia.
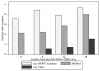
Results: Between 2002 and 2006, 3727 patients initiated cART (median CD4, 147 cells/mm(3)). Of 1007 patients who developed viremia, 815 had subsequent HIV RNA assays, and 331 (41%) of these resuppressed without regimen switch. At identification of viremia, 45 (66%) of 68 patients had HIV-1 drug resistance, 42 (62%) had nonnucleoside reverse-transcriptase inhibitor (NNRTI)-resistance, 25 (37%) had M184V/I, and 4 (6%) had multi-nucleoside analogue drug mutations. By 12 months of persistent viremia among a subset of 14 patients with resistance testing to 12 months, 11 (78%) had nonnucleoside reverse-transcriptase inhibitor (NNRTI)-resistance, 8 (57%) had M184V/I, and 2 (14%) had multi-nucleoside analogue drug mutations. Resistance was associated with a reduced probability of resuppression; however, 50% of patients with NNRTI resistance resuppressed while receiving an NNRTI.
Conclusions: The majority of patients had NNRTI resistance mutations at detection of viremia. However, 41% resuppressed without regimen switch. Our findings support maximizing first-line use while minimizing risk of significant cross-resistance by implementing intensive adherence support and repeat HIV RNA testing 3-6 months after detecting viremia, with regimen switch only if viremia persists.
Conflict of interest statement
Figures
References
-
- Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. - PubMed
-
- Virological response to a triple nucleoside/nucleotide analogue regimen over 48 weeks in HIV-1-infected adults in Africa. AIDS. 2006;20:1391–9. - PubMed
-
- Bekker LG, Myer L, Orrell C, Lawn S, Wood R. Rapid scale-up of a community-based HIV treatment service: programme performance over 3 consecutive years in Guguletu, South Africa. S Afr Med J. 2006;96:315–20. - PubMed
-
- Ramadhani HO, Thielman NM, Landman KZ, et al. Predictors of incomplete adherence, virologic failure, and antiviral drug resistance among HIV-infected adults receiving antiretroviral therapy in Tanzania. Clin Infect Dis. 2007;45:1492–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

