Gene expression analysis of tuberous sclerosis complex cortical tubers reveals increased expression of adhesion and inflammatory factors
- PMID: 19912235
- PMCID: PMC2888867
- DOI: 10.1111/j.1750-3639.2009.00341.x
Gene expression analysis of tuberous sclerosis complex cortical tubers reveals increased expression of adhesion and inflammatory factors
Abstract
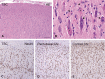
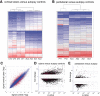
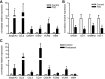
Cortical tubers in patients with tuberous sclerosis complex are associated with disabling neurological manifestations, including intractable epilepsy. While these malformations are believed to result from the effects of TSC1 or TSC2 gene mutations, the molecular mechanisms leading to tuber formation, as well as the onset of seizures, remain largely unknown. We used the Affymetrix Gene Chip platform to provide the first genome-wide investigation of gene expression in surgically resected tubers, compared with histological normal perituberal tissue from the same patients or autopsy control tissue. We identified 2501 differentially expressed genes in cortical tubers compared with autopsy controls. Expression of genes associated with cell adhesion, for example, VCAM1, integrins and CD44, or with the inflammatory response, including complement factors, serpinA3, CCL2 and several cytokines, was increased in cortical tubers, whereas genes related to synaptic transmission, for example, the glial glutamate transporter GLT-1, and voltage-gated channel activity, exhibited lower expression. Gene expression in perituberal cortex was distinct from autopsy control cortex suggesting that even in the absence of tissue pathology the transcriptome is altered in TSC. Changes in gene expression yield insights into new candidate genes that may contribute to tuber formation or seizure onset, representing new targets for potential therapeutic development.
Figures

References
-
- Arai Y, Takashima S, Becker LE (2000) Cd44 expression in tuberous sclerosis. Pathobiology 68:87–92. - PubMed
-
- Aronica E, Boer K, Becker A, Redeker S, Spliet WG, Van Rijen PC et al (2008) Gene expression profile analysis of epilepsy‐associated gangliogliomas. Neuroscience 151:272–292. - PubMed
-
- Aronica E, Gorter J (2007) Gene expression profile in temporal lobe epilepsy. Neuroscientist 13:1–9. - PubMed
-
- Aronica E, Gorter JA, Redeker S, Ramkema M, Spliet WG, Van Rijen PC et al (2005) Distribution, characterization and clinical significance of microglia in glioneuronal tumours from patients with chronic intractable epilepsy. Neuropathol Appl Neurobiol 31:280–291. - PubMed
-
- Aronica E, Redeker S, Boer K, Spliet WG, Van Rijen PC, Gorter JA, Troost D (2007) Inhibitory networks in epilepsy‐associated gangliogliomas and in the perilesional epileptic cortex. Epilepsy Res 74:33–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

