Translational mini-review series on Th17 cells: induction of interleukin-17 production by regulatory T cells
- PMID: 19912251
- PMCID: PMC2810380
- DOI: 10.1111/j.1365-2249.2009.04038.x
Translational mini-review series on Th17 cells: induction of interleukin-17 production by regulatory T cells
Abstract
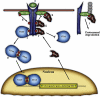
Uncommitted (naive) CD4(+) T helper cells (Thp) can be induced to differentiate to specific lineages according to the local cytokine milieu, towards T helper type 1 (Th1), Th2, Th17 and regulatory T cell (T(reg)) phenotypes in a mutually exclusive manner. Each phenotype is characterized by unique signalling pathways and expression of specific transcription factors, notably T-bet for Th1, GATA-3 for Th2, forkhead box P3 (FoxP3) for T(regs) and receptor-related orphan receptor (ROR)alpha and RORgammat for Th17 cells. T(regs) and Th17 cells have been demonstrated to arise from common precursors in a reciprocal manner based on exposure to transforming growth factor (TGF)-beta or TGF-beta plus interleukin (IL)-6 and carry out diametrically opposing functions, namely suppression or propagation of inflammation, respectively. However, while epigenetic modifications in Th1 and Th2 differentiated cells prevents their conversion to other phenotypes, Th17 cells generated in vitro using TGF-beta and IL-6 are unstable and can convert to other phenotypes, especially Th1, both in vitro and in vivo. T(regs) are generated from naive precursors both in the thymus (natural, nT(regs)) and in the periphery (induced, iT(regs)). The highly suppressive function of T(regs) enables them to control many inflammatory diseases in animals and makes them particularly attractive candidates for immunotherapy in humans. The stability of the T(reg) phenotype is therefore of paramount importance in this context. Recent descriptions of T(reg) biology have suggested that components of pathogens or inflammatory mediators may subvert the suppressive function of T(regs) in order to allow propagation of adequate immune responses. Unexpectedly, however, a number of groups have now described conversion of T(regs) to the Th17 phenotype induced by appropriate inflammatory stimuli. These observations are particularly relevant in the context of cell therapy but may also explain some of the dysregulation seen in autoimmune diseases. In this paper, we review T(reg) to Th17 conversion and propose some potential mechanisms for this phenomenon.
Figures

References
-
- Palmer E. Negative selection – clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol. 2003;3:383–91. - PubMed
-
- Grazia Roncarolo M, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. - PubMed
-
- Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

