Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV)
- PMID: 19912623
- PMCID: PMC2785784
- DOI: 10.1186/1743-422X-6-197
Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV)
Abstract
Background: Influenza virus (IV) infections are a major threat to human welfare and animal health worldwide. Anti-viral therapy includes vaccines and a few anti-viral drugs. However vaccines are not always available in time, as demonstrated by the emergence of the new 2009 H1N1-type pandemic strain of swine origin (S-OIV) in April 2009, and the acquisition of resistance to neuraminidase inhibitors such as Tamiflu (oseltamivir) is a potential problem. Therefore the prospects for the control of IV by existing anti-viral drugs are limited. As an alternative approach to the common anti-virals we studied in more detail a commercial standardized extract of the widely used herb Echinacea purpurea (Echinaforce, EF) in order to elucidate the nature of its anti-IV activity.
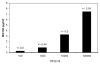
Results: Human H1N1-type IV, highly pathogenic avian IV (HPAIV) of the H5- and H7-types, as well as swine origin IV (S-OIV, H1N1), were all inactivated in cell culture assays by the EF preparation at concentrations ranging from the recommended dose for oral consumption to several orders of magnitude lower. Detailed studies with the H5N1 HPAIV strain indicated that direct contact between EF and virus was required, prior to infection, in order to obtain maximum inhibition in virus replication. Hemagglutination assays showed that the extract inhibited the receptor binding activity of the virus, suggesting that the extract interferes with the viral entry into cells. In sequential passage studies under treatment in cell culture with the H5N1 virus no EF-resistant variants emerged, in contrast to Tamiflu, which produced resistant viruses upon passaging. Furthermore, the Tamiflu-resistant virus was just as susceptible to EF as the wild type virus.
Conclusion: As a result of these investigations, we believe that this standard Echinacea preparation, used at the recommended dose for oral consumption, could be a useful, readily available and affordable addition to existing control options for IV replication and dissemination.
Figures
References
-
- Suzuki Y. The Highly Pathogenic Avian Influenza H5N1-Initial Molecular Signals for the Next Influenza Pandemic. Chang Gung Med J. 2009;32:258–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

