Mapping interaction sites within the N-terminus of the calcitonin gene-related peptide receptor; the role of residues 23-60 of the calcitonin receptor-like receptor
- PMID: 19913063
- PMCID: PMC2809212
- DOI: 10.1016/j.peptides.2009.10.021
Mapping interaction sites within the N-terminus of the calcitonin gene-related peptide receptor; the role of residues 23-60 of the calcitonin receptor-like receptor
Abstract
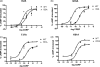
The calcitonin receptor-like receptor (CLR) acts as a receptor for the calcitonin gene-related peptide (CGRP) but in order to recognize CGRP, it must form a complex with an accessory protein, receptor activity modifying protein 1 (RAMP1). Identifying the protein/protein and protein/ligand interfaces in this unusual complex would aid drug design. The role of the extreme N-terminus of CLR (Glu23-Ala60) was examined by an alanine scan and the results were interpreted with the help of a molecular model. The potency of CGRP at stimulating cAMP production was reduced at Leu41Ala, Gln45Ala, Cys48Ala and Tyr49Ala; furthermore, CGRP-induced receptor internalization at all of these receptors was also impaired. Ile32Ala, Gly35Ala and Thr37Ala all increased CGRP potency. CGRP specific binding was abolished at Leu41Ala, Ala44Leu, Cys48Ala and Tyr49Ala. There was significant impairment of cell surface expression of Gln45Ala, Cys48Ala and Tyr49Ala. Cys48 takes part in a highly conserved disulfide bond and is probably needed for correct folding of CLR. The model suggests that Gln45 and Tyr49 mediate their effects by interacting with RAMP1 whereas Leu41 and Ala44 are likely to be involved in binding CGRP. Ile32, Gly35 and Thr37 form a separate cluster of residues which modulate CGRP binding. The results from this study may be applicable to other family B GPCRs which can associate with RAMPs.
Figures
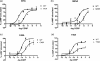

References
-
- Aiyar N., Daines R.A., Disa J., Chambers P.A., Sauermelch C.F., Quiniou M. Pharmacology of SB-273779, a nonpeptide calcitonin gene-related peptide 1 receptor antagonist. J Pharmacol Exp Ther. 2001;296:768–775. - PubMed
-
- Christopoulos A., Christopoulos G., Morfis M., Udawela M., Laburthe M., Couvineau A. Novel receptor partners and function of receptor activity-modifying proteins. J Biol Chem. 2003;278:3293–3297. - PubMed
-
- Christopoulos G., Perry K.J., Morfis M., Tilakaratne N., Gao Y., Fraser N.J. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol. 1999;56:235–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

