The molecular clockwork of a protein-based circadian oscillator
- PMID: 19913541
- PMCID: PMC2810098
- DOI: 10.1016/j.febslet.2009.11.021
The molecular clockwork of a protein-based circadian oscillator
Abstract
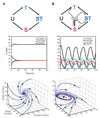
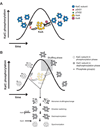
The circadian clock of the cyanobacterium Synechococcus elongatus PCC 7942 is governed by a core oscillator consisting of the proteins KaiA, KaiB, and KaiC. Remarkably, circadian oscillations in the phosphorylation state of KaiC can be reconstituted in a test tube by mixing the three Kai proteins and adenosine triphosphate. The in vitro oscillator provides a well-defined system in which experiments can be combined with mathematical analysis to understand the mechanism of a highly robust biological oscillator. In this Review, we summarize the biochemistry of the Kai proteins and examine models that have been proposed to explain how oscillations emerge from the properties of the oscillator's constituents.
Figures


References
-
- Dunlap JC, Loros JJ, DeCoursey PJ. Chronobiology: biological timekeeping. Sunderland, MA: Sinauer Associates; 2004.
-
- Tomita J, Nakajima M, Kondo T, Iwasaki H. No transcription-translation feedback in circadian rhythm of KaiC phosphorylation. Science. 2005;307:251–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

