Gene manipulated peritoneal cell patch repairs infarcted myocardium
- PMID: 19913551
- PMCID: PMC2905838
- DOI: 10.1016/j.yjmcc.2009.10.032
Gene manipulated peritoneal cell patch repairs infarcted myocardium
Abstract
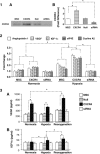
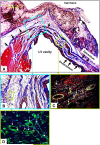
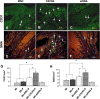
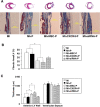
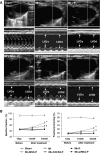
A gene manipulated cell patch using a homologous peritoneum substrate was developed and applied after myocardial infarction to repair scarred myocardium. We genetically engineered male rat mesenchymal stem cells (MSC) using adenoviral transduction to over-express CXCR4/green fluorescent protein (GFP) (MSC(CXCR4)) or MSC(Null) or siRNA targeting CXCR4 (MSC(siRNA)). Gene expression was studied by real-time quantitative PCR (qPCR) and enzyme-linked immunosorbent assay (ELISA). Cells were cultured on excised peritoneum for 9 days. Two weeks after left anterior descending (LAD) coronary artery ligation in female hearts, the peritoneum patch was applied over the scarred myocardium, cell side down. Efficacy of engraftment was determined by presence of GFP positive cells. One month after cell implantation, echocardiography was performed and hearts were harvested for histological analysis. Left ventricle (LV) fibrosis, LV anterior wall thickness (AWT) and blood vessel density at the margins of the graft were measured. There was significant up-regulation of the chemokines in the MSC(CXCR4) group cultured under normoxic conditions when compared to the MSC(Null) group and a further increase was observed after exposure to hypoxia. One month after cell transplantation with the peritoneum patch, substantial numbers of GFP-positive cells were observed in and around the infarcted myocardium in MSC(CXCR4) group. LV AWT, LV fibrosis and LV function were significantly improved in the MSC(CXCR4) group as compared to these same variables in the MSC(Null) control. These salutary effects were absent in MSC(siRNA) group. The gene manipulated MSC-seeded peritoneum patch promotes tissue nutrition (angiogenesis), reduces myocardial remodeling, and enhances heart function after myocardial infarction.
Copyright (c) 2009 Elsevier Ltd. All rights reserved.
Figures


References
-
- Almsherqi ZA, McLachlan CS, Slocinska MB, Sluse FE, Navet R, Kocherginsky N, et al. Reduced cardiac output is associated with decreased mitochondrial efficiency in the non-ischemic ventricular wall of the acute myocardial-infarcted dog. Cell Res. 2006;16:297–305. - PubMed
-
- O'Leary DP. Use of the greater omentum in colorectal surgery. Dis Colon Rectum. 1999;42:533–9. - PubMed
-
- O'Shaughnessy L. Surgical treatment of cardiac ischemia. Lancet. 1937;232:185–94.
-
- Suzuki R, Hattori F, Itabashi Y, Yoshioka M, Yuasa S, Manabe-Kawaguchi H, et al. Omentopexy enhances graft function in myocardial cell sheet transplantation. Biochem Biophys Res Commun. 2009;387:353–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

