Dysregulation of sterol regulatory element binding protein-1c in livers of morbidly obese women is associated with altered suppressor of cytokine signaling-3 and signal transducer and activator of transcription-1 signaling
- PMID: 19913854
- PMCID: PMC2843778
- DOI: 10.1016/j.metabol.2009.09.001
Dysregulation of sterol regulatory element binding protein-1c in livers of morbidly obese women is associated with altered suppressor of cytokine signaling-3 and signal transducer and activator of transcription-1 signaling
Abstract
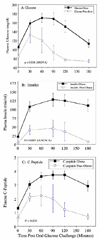
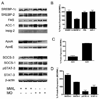
We compared hepatic expression of genes that regulate lipid biosynthesis and metabolic signaling in liver biopsy specimens from women who were undergoing gastric bypass surgery (GBP) for morbid obesity with that in women undergoing ventral hernia repair who had experienced massive weight loss (MWL) after prior GBP. Comprehensive metabolic profiles of morbidly obese (MO) (22 subjects) and MWL (9 subjects) were also compared. Analyses of gene expression in liver biopsies from MO and MWL were accomplished by Affymetrix microarray, real-time polymerase chain reaction, and Western blotting techniques. After GBP, MWL subjects had lost on average 102 lb as compared with MO subjects. This was accompanied by effective reversal of the dyslipidemia and insulin resistance that were present in MO. As compared with MWL, livers of MO subjects exhibited increased expression of sterol regulatory element binding protein (SREBP)-1c and its downstream lipogenic targets, fatty acid synthase and acetyl-coenzyme A-carboxylase-1. Livers of MO subjects also exhibited enhanced expression of suppressor of cytokine signaling-3 protein and attenuated Janus kinase signal transducer and activator of transcription (JAK/STAT) signaling. Consistent with these findings, we found that the human SREBP-1c promoter was positively regulated by insulin and negatively regulated by STAT3. These data support the hypothesis that suppressor of cytokine signaling-3-mediated attenuation of the STAT signaling pathway and resulting enhanced expression of SREBP-1c, a key regulator of de novo lipid biosynthesis, are mechanistically related to the development of hepatic insulin resistance and dyslipidemia in MO women.
Published by Elsevier Inc.
Conflict of interest statement
Figures


Similar articles
-
Hepatic gene expression in morbidly obese women: implications for disease susceptibility.Obesity (Silver Spring). 2009 Aug;17(8):1563-73. doi: 10.1038/oby.2009.49. Epub 2009 Mar 5. Obesity (Silver Spring). 2009. PMID: 19265796
-
Liver-specific expression of transcriptionally active SREBP-1c is associated with fatty liver and increased visceral fat mass.PLoS One. 2012;7(2):e31812. doi: 10.1371/journal.pone.0031812. Epub 2012 Feb 21. PLoS One. 2012. PMID: 22363740 Free PMC article.
-
Cilostazol inhibits insulin-stimulated expression of sterol regulatory binding protein-1c via inhibition of LXR and Sp1.Exp Mol Med. 2014 Jan 24;46(1):e73. doi: 10.1038/emm.2013.143. Exp Mol Med. 2014. PMID: 24458133 Free PMC article.
-
SREBP-1c and TFE3, energy transcription factors that regulate hepatic insulin signaling.J Mol Med (Berl). 2007 May;85(5):437-44. doi: 10.1007/s00109-007-0158-5. Epub 2007 Feb 6. J Mol Med (Berl). 2007. PMID: 17279346 Review.
-
Sterol regulatory element-binding protein family as global regulators of lipid synthetic genes in energy metabolism.Vitam Horm. 2002;65:167-94. doi: 10.1016/s0083-6729(02)65064-2. Vitam Horm. 2002. PMID: 12481547 Review.
Cited by
-
Catalpol Attenuates Hepatic Steatosis by Regulating Lipid Metabolism via AMP-Activated Protein Kinase Activation.Biomed Res Int. 2020 Apr 25;2020:6708061. doi: 10.1155/2020/6708061. eCollection 2020. Biomed Res Int. 2020. PMID: 32420361 Free PMC article.
-
New insights into the mechanism of low high-density lipoprotein cholesterol in obesity.Lipids Health Dis. 2011 Oct 12;10:176. doi: 10.1186/1476-511X-10-176. Lipids Health Dis. 2011. PMID: 21988829 Free PMC article. Review.
-
Nuclear receptors reverse McGarry's vicious cycle to insulin resistance.Cell Metab. 2012 May 2;15(5):615-22. doi: 10.1016/j.cmet.2012.03.016. Cell Metab. 2012. PMID: 22560214 Free PMC article.
-
SOCS3-mediated blockade reveals major contribution of JAK2/STAT5 signaling pathway to lactation and proliferation of dairy cow mammary epithelial cells in vitro.Molecules. 2013 Oct 17;18(10):12987-3002. doi: 10.3390/molecules181012987. Molecules. 2013. PMID: 24141248 Free PMC article.
-
Suppressor of Cytokine Signaling-3 (SOCS-3) Induces Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Expression in Hepatic HepG2 Cell Line.J Biol Chem. 2016 Feb 12;291(7):3508-19. doi: 10.1074/jbc.M115.664706. Epub 2015 Dec 14. J Biol Chem. 2016. PMID: 26668321 Free PMC article.
References
-
- Grundy SM. Metabolic complications of obesity. Endocrine. 2000 Oct;13(2):155–165. - PubMed
-
- Castro Cabezas M, Halkes CJ, Erkelens DW. Obesity and free fatty acids: double trouble. Nutr Metab Cardiovasc Dis. 2001 Apr;11(2):134–142. - PubMed
-
- Festi D, Colecchia A, Sacco Sacco T, Bondi M, Roda E, Marchesini G. Hepatic steatosis in obese patients: clinical aspects and prognostic significance. Obes Rev. 2004 Feb;5(1):27–42. - PubMed
-
- Marchesini G, Marzocchi R, Agostini F, Bugianesi E. Nonalcoholic fatty liver disease and the metabolic syndrome. Curr Opin Lipidol. 2005 Aug;16(4):421–427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

