Alternate receptor usage of neuropilin-1 and glucose transporter protein 1 by the human T cell leukemia virus type 1
- PMID: 19913864
- PMCID: PMC2789895
- DOI: 10.1016/j.virol.2009.10.015
Alternate receptor usage of neuropilin-1 and glucose transporter protein 1 by the human T cell leukemia virus type 1
Abstract
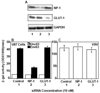
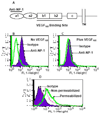
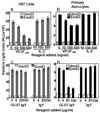
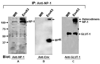
Recent studies have demonstrated that neuropilin 1 (NP-1) is involved in HTLV-1 entry; however, the role NP-1 plays in this process is not understood. We demonstrated that ectopic expression of human NP-1 but not NP-2 cDNA increased susceptibility to HTLV-1. SiRNA-mediated inhibition of NP-1 expression correlated with significant reduction of HTLV-1 Env-mediated fusion. The vascular endothelial growth factor (VEGF(165)) caused downmodulation of surface NP-1 and inhibited HTLV-1 infection of U87 cells. In contrast, VEGF(165) partially inhibited infection of primary astrocytes and had no significant effect on infection of HeLa cells. VEGF(165) and antibodies to the glucose transporter protein 1 (anti-GLUT-1) were both needed to block infection of primary astrocytes, however, only anti-GLUT-1 antibodies were sufficient to block infection of HeLa cells. HTLV-1 Env forms complexes with both NP-1 and GLUT-1 in primary human astrocytes. The alternate usage of these two cellular receptors may have important implications regarding HTLV-1 neuro-tropism.
Figures



Similar articles
-
Neuropilin-1 is involved in human T-cell lymphotropic virus type 1 entry.J Virol. 2006 Jul;80(14):6844-54. doi: 10.1128/JVI.02719-05. J Virol. 2006. PMID: 16809290 Free PMC article.
-
GLUT-1-independent infection of the glioblastoma/astroglioma U87 cells by the human T cell leukemia virus type 1.Virology. 2006 Sep 15;353(1):99-110. doi: 10.1016/j.virol.2006.05.003. Epub 2006 Jun 16. Virology. 2006. PMID: 16781755
-
Human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 use different receptor complexes to enter T cells.J Virol. 2006 Sep;80(17):8291-302. doi: 10.1128/JVI.00389-06. J Virol. 2006. PMID: 16912281 Free PMC article.
-
Interaction between the HTLV-1 envelope and cellular proteins: impact on virus infection and restriction.Future Med Chem. 2010 Nov;2(11):1651-68. doi: 10.4155/fmc.10.255. Future Med Chem. 2010. PMID: 21428837 Review.
-
Molecular aspects of HTLV-1 entry: functional domains of the HTLV-1 surface subunit (SU) and their relationships to the entry receptors.Viruses. 2011 Jun;3(6):794-810. doi: 10.3390/v3060794. Epub 2011 Jun 15. Viruses. 2011. PMID: 21994754 Free PMC article. Review.
Cited by
-
Cellular Factors Involved in HTLV-1 Entry and Pathogenicit.Front Microbiol. 2012 Jun 21;3:222. doi: 10.3389/fmicb.2012.00222. eCollection 2012. Front Microbiol. 2012. PMID: 22737146 Free PMC article.
-
Neuropilins: expression and roles in the epithelium.Int J Exp Pathol. 2012 Apr;93(2):81-103. doi: 10.1111/j.1365-2613.2012.00810.x. Int J Exp Pathol. 2012. PMID: 22414290 Free PMC article. Review.
-
Human T-cell leukemia virus type 1 Tax-deregulated autophagy pathway and c-FLIP expression contribute to resistance against death receptor-mediated apoptosis.J Virol. 2014 Mar;88(5):2786-98. doi: 10.1128/JVI.03025-13. Epub 2013 Dec 18. J Virol. 2014. PMID: 24352466 Free PMC article.
-
Functional analyse of GLUT1 and GLUT12 in glucose uptake in goat mammary gland epithelial cells.PLoS One. 2013 May 28;8(5):e65013. doi: 10.1371/journal.pone.0065013. Print 2013. PLoS One. 2013. PMID: 23724114 Free PMC article.
-
Does HTLV-1 Infection Show Phenotypes Found in Sjögren's Syndrome?Viruses. 2022 Jan 6;14(1):100. doi: 10.3390/v14010100. Viruses. 2022. PMID: 35062304 Free PMC article. Review.
References
-
- Agrawal L, Lu X, Jin Q, Alkhatib G. Anti-HIV Therapy: Current and Future Directions. Curr Pharm Des. 2006;12(16):2031–2055. - PubMed
-
- Agrawal L, Vanhorn-Ali Z, Alkhatib G. Multiple determinants are involved in HIV coreceptor use as demonstrated by CCR4/CCL22 interaction in peripheral blood mononuclear cells (PBMCs) J Leukoc Biol. 2002;72(5):1063–1074. - PubMed
-
- Agrawal L, VanHorn-Ali Z, Berger EA, Alkhatib G. Specific inhibition of HIV-1 coreceptor activity by synthetic peptides corresponding to the predicted extracellular loops of CCR5. Blood. 2004b;103(4):1211–1217. - PubMed
-
- Altmeyer R. Virus attachment and entry offer numerous targets for antiviral therapy. Curr Pharm Des. 2004;10(30):3701–3712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

