Structural convergence between Cryo-EM and NMR reveals intersubunit interactions critical for HIV-1 capsid function
- PMID: 19914170
- PMCID: PMC2782912
- DOI: 10.1016/j.cell.2009.10.010
Structural convergence between Cryo-EM and NMR reveals intersubunit interactions critical for HIV-1 capsid function
Abstract
Mature HIV-1 particles contain conical-shaped capsids that enclose the viral RNA genome and perform essential functions in the virus life cycle. Previous structural analysis of two- and three-dimensional arrays of the capsid protein (CA) hexamer revealed three interfaces. Here, we present a cryoEM study of a tubular assembly of CA and a high-resolution NMR structure of the CA C-terminal domain (CTD) dimer. In the solution dimer structure, the monomers exhibit different relative orientations compared to previous X-ray structures. The solution structure fits well into the EM density map, suggesting that the dimer interface is retained in the assembled CA. We also identified a CTD-CTD interface at the local three-fold axis in the cryoEM map and confirmed its functional importance by mutagenesis. In the tubular assembly, CA intermolecular interfaces vary slightly, accommodating the asymmetry present in tubes. This provides the necessary plasticity to allow for controlled virus capsid dis/assembly.
Figures


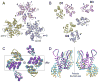


References
-
- Bax A, Grzesiek S. Methodological advances in protein NMR. Acc Chem Res. 1993;26:131–138.
-
- Bosco DA, Kern D. Catalysis and binding of cyclophilin A with different HIV-1 capsid constructs. Biochemistry. 2004;43:6110–6119. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

