Extracellular Engrailed participates in the topographic guidance of retinal axons in vivo
- PMID: 19914184
- PMCID: PMC4603356
- DOI: 10.1016/j.neuron.2009.09.018
Extracellular Engrailed participates in the topographic guidance of retinal axons in vivo
Abstract
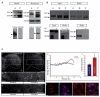
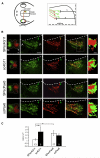
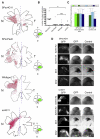
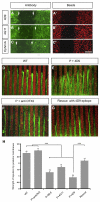
Engrailed transcription factors regulate the expression of guidance cues that pattern retinal axon terminals in the dorsal midbrain. They also act directly to guide axon growth in vitro. We show here that an extracellular En gradient exists in the tectum along the anterior-posterior axis. Neutralizing extracellular Engrailed in vivo with antibodies expressed in the tectum causes temporal axons to map aberrantly to the posterior tectum in chick and Xenopus. Furthermore, posterior membranes from wild-type tecta incubated with anti-Engrailed antibodies or posterior membranes from Engrailed-1 knockout mice exhibit diminished repulsive activity for temporal axons. Since EphrinAs play a major role in anterior-posterior mapping, we tested whether Engrailed cooperates with EphrinA5 in vitro. We find that Engrailed restores full repulsion to axons given subthreshold doses of EphrinA5. Collectively, our results indicate that extracellular Engrailed contributes to retinotectal mapping in vivo by modulating the sensitivity of growth cones to EphrinA.
Figures
Similar articles
-
Target-independent ephrina/EphA-mediated axon-axon repulsion as a novel element in retinocollicular mapping.Neuron. 2014 Nov 19;84(4):740-52. doi: 10.1016/j.neuron.2014.09.023. Epub 2014 Oct 23. Neuron. 2014. PMID: 25451192 Free PMC article.
-
Topographic-specific axon branching controlled by ephrin-As is the critical event in retinotectal map development.J Neurosci. 2001 Nov 1;21(21):8548-63. doi: 10.1523/JNEUROSCI.21-21-08548.2001. J Neurosci. 2001. PMID: 11606643 Free PMC article.
-
Retroviral misexpression of engrailed genes in the chick optic tectum perturbs the topographic targeting of retinal axons.J Neurosci. 1996 Sep 1;16(17):5498-509. doi: 10.1523/JNEUROSCI.16-17-05498.1996. J Neurosci. 1996. PMID: 8757262 Free PMC article.
-
Key roles of Ephs and ephrins in retinotectal topographic map formation.Brain Res Bull. 2009 Jun 30;79(5):227-47. doi: 10.1016/j.brainresbull.2009.03.008. Epub 2009 Apr 1. Brain Res Bull. 2009. PMID: 19480983 Review.
-
Engrailed homeoproteins in visual system development.Cell Mol Life Sci. 2015 Apr;72(8):1433-45. doi: 10.1007/s00018-014-1776-z. Epub 2014 Nov 29. Cell Mol Life Sci. 2015. PMID: 25432704 Free PMC article. Review.
Cited by
-
How to navigate counter dogmatic research findings.EMBO J. 2018 Feb 15;37(4):e98945. doi: 10.15252/embj.201898945. Epub 2018 Jan 17. EMBO J. 2018. PMID: 29343544 Free PMC article.
-
Non-cell-autonomous OTX2 transcription factor regulates anxiety-related behavior in the mouse.Mol Psychiatry. 2021 Nov;26(11):6469-6480. doi: 10.1038/s41380-021-01132-y. Epub 2021 May 7. Mol Psychiatry. 2021. PMID: 33963285 Free PMC article.
-
The Nuclear Transitory Zone: A Key Player in the Cerebellar Development.Cerebellum. 2025 May 2;24(4):92. doi: 10.1007/s12311-025-01848-5. Cerebellum. 2025. PMID: 40314748 Review.
-
Signaling with homeoprotein transcription factors in development and throughout adulthood.Curr Genomics. 2013 Sep;14(6):361-70. doi: 10.2174/1389202911314060009. Curr Genomics. 2013. PMID: 24396269 Free PMC article.
-
Local translation of mRNAs in neural development.Wiley Interdiscip Rev RNA. 2011 Jan-Feb;2(1):153-65. doi: 10.1002/wrna.53. Epub 2010 Oct 19. Wiley Interdiscip Rev RNA. 2011. PMID: 21956974 Free PMC article. Review.
References
-
- Auerbach R, Kubai L, Knighton D, Folkman J. A simple procedure for the long-term cultivation of chicken embryos. Dev Biol. 1974;41:391–394. - PubMed
-
- Auf der Maur A, Tissot K, Barberis A. Antigen-independent selection of intracellular stable antibody frameworks. Methods. 2004;34:215–224. - PubMed
-
- Bovolenta P. Morphogen signaling at the vertebrate growth cone: a few cases or a general strategy? J Neurobiol. 2005;64:405–416. - PubMed
-
- Brunet I, Di Nardo AA, Sonnier L, Beurdeley M, Prochiantz A. The topological role of homeoproteins in the developing central nervous system. Trends in Neurosci. 2007 In press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
