Toll-like receptor 2 is required for opioids-induced neuronal apoptosis
- PMID: 19914204
- PMCID: PMC2812588
- DOI: 10.1016/j.bbrc.2009.11.074
Toll-like receptor 2 is required for opioids-induced neuronal apoptosis
Abstract
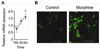
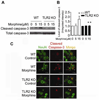
Toll-like receptor 2 (TLR2), a key immune receptor in the TLR family, is widely expressed in various systems, including the immune and nervous systems and plays a critical role in controlling innate and adaptive immune responses. We previously reported that opioids inhibit cell growth and trigger apoptosis. However, the underlying mechanism by which TLR2 mediates apoptosis in response to opioids is not yet known. Here we show that chronic morphine treatment in primary neurons dramatically increased the expression of TLR2 at both the messenger RNA and protein levels. In addition, TLR2 deficiency significantly inhibited chronic morphine-induced apoptosis in primary neurons. Activation of caspase-3 after morphine treatment is impaired in TLR2 deficient primary neurons. Moreover, morphine treatment failed to induce an increased level of phosphorylated glycogen synthase kinase 3 beta (GSK3beta) in TLR2 deficient primary neurons, suggesting an involvement of GSK3beta in morphine-mediated TLR2 signaling. These results thus demonstrate that opioids prime neurons to undergo apoptosis by inducing TLR2 expression. Our data suggest that inhibition of TLR2 is capable of preventing opioids-induced damage to neurons.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures
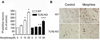

Similar articles
-
Glycogen synthase kinase-3 and p38 MAPK are required for opioid-induced microglia apoptosis.Neuropharmacology. 2010 Nov;59(6):444-51. doi: 10.1016/j.neuropharm.2010.06.006. Epub 2010 Jun 22. Neuropharmacology. 2010. PMID: 20600172 Free PMC article.
-
The JAK2-Akt-glycogen synthase kinase-3β signaling pathway is involved in toll-like receptor 2-induced monocyte chemoattractant protein-1 regulation.Mol Med Rep. 2012 Apr;5(4):1063-7. doi: 10.3892/mmr.2012.741. Epub 2012 Jan 3. Mol Med Rep. 2012. PMID: 22218715 Free PMC article.
-
Toll-like receptor 9 is required for opioid-induced microglia apoptosis.PLoS One. 2011 Apr 29;6(4):e18190. doi: 10.1371/journal.pone.0018190. PLoS One. 2011. PMID: 21559519 Free PMC article.
-
Essential role of toll-like receptor 2 in morphine-induced microglia activation in mice.Neurosci Lett. 2011 Feb 1;489(1):43-7. doi: 10.1016/j.neulet.2010.11.063. Epub 2010 Dec 2. Neurosci Lett. 2011. PMID: 21130144 Free PMC article.
-
Synthetic resveratrol aliphatic acid inhibits TLR2-mediated apoptosis and an involvement of Akt/GSK3beta pathway.Bioorg Med Chem. 2009 Jul 1;17(13):4378-82. doi: 10.1016/j.bmc.2009.05.029. Epub 2009 May 18. Bioorg Med Chem. 2009. PMID: 19477653 Free PMC article.
Cited by
-
Opiate drug use and the pathophysiology of neuroAIDS.Curr HIV Res. 2012 Jul;10(5):435-52. doi: 10.2174/157016212802138779. Curr HIV Res. 2012. PMID: 22591368 Free PMC article. Review.
-
Morphine enhances LPS-induced macrophage apoptosis through a PPARγ-dependent mechanism.Exp Ther Med. 2021 Jul;22(1):714. doi: 10.3892/etm.2021.10146. Epub 2021 May 3. Exp Ther Med. 2021. PMID: 34007323 Free PMC article.
-
GSK3β-activation is a point of convergence for HIV-1 and opiate-mediated interactive neurotoxicity.Mol Cell Neurosci. 2015 Mar;65:11-20. doi: 10.1016/j.mcn.2015.01.001. Epub 2015 Jan 20. Mol Cell Neurosci. 2015. PMID: 25616162 Free PMC article.
-
Morphine and HIV-1 gp120 cooperatively promote pathogenesis in the spinal pain neural circuit.Mol Pain. 2019 Jan-Dec;15:1744806919868380. doi: 10.1177/1744806919868380. Mol Pain. 2019. PMID: 31368399 Free PMC article.
-
Toll-like receptor 9 is required for chronic stress-induced immune suppression.Neuroimmunomodulation. 2014;21(1):1-7. doi: 10.1159/000354610. Epub 2013 Sep 24. Neuroimmunomodulation. 2014. PMID: 24080854 Free PMC article.
References
-
- Yin D, Mufson RA, Wang R, Shi Y. Fas-mediated cell death promoted by opioids. Nature. 1999;397:218. - PubMed
-
- Yin D, Woodruff M, Zhang Y, Whaley S, Miao J, Ferslew K, Zhao J, Stuart C. Morphine promotes Jurkat cell apoptosis through pro-apoptotic FADD/P53 and anti-apoptotic PI3K/Akt/NF-kappaB pathways. J.Neuroimmunol. 2006;174:101–107. - PubMed
-
- Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–836. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

