Gbb/BMP signaling is required to maintain energy homeostasis in Drosophila
- PMID: 19914231
- PMCID: PMC2838617
- DOI: 10.1016/j.ydbio.2009.11.011
Gbb/BMP signaling is required to maintain energy homeostasis in Drosophila
Abstract
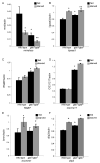
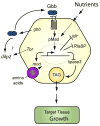
The coordination of animal growth and development requires adequate nutrients. During times of insufficient food, developmental progression is slowed and stored energy is utilized to ensure that cell and tissue survival are maintained. Here, we report our finding that the Gbb/BMP signaling pathway, known to play an important role in many developmental processes in both vertebrates and invertebrates, is critical in the Drosophila larval fat body for regulating energy homeostasis. Animals with mutations in the Drosophila BMP-5,7 orthologue, glass bottom boat (gbb), or in its signaling components, display phenotypes similar to nutrient-deprived and Tor mutant larvae. These phenotypes include a developmental delay with reduced overall growth, a transparent appearance, and altered total lipid, glucose and trehalose levels. We find that Gbb/BMP signaling is required in the larval fat body for maintaining proper metabolism, yet interestingly, following nutrient deprivation larvae in turn show a loss of BMP signaling in fat body cells indicating that Gbb/BMP signaling is a central player in homeostasis. Finally, despite strong phenotypic similarities between nutrient-compromised animals and gbb mutants, distinct differences are observed in the expression of a group of starvation responsive genes. Overall, our results implicate Gbb/BMP signaling as a new pathway critical for positive regulation of nutrient storage and energy homeostasis during development.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures

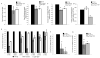


Similar articles
-
Crimpy inhibits the BMP homolog Gbb in motoneurons to enable proper growth control at the Drosophila neuromuscular junction.Development. 2011 Aug;138(15):3273-86. doi: 10.1242/dev.066142. Development. 2011. PMID: 21750037 Free PMC article.
-
Alternative cleavage of the bone morphogenetic protein (BMP), Gbb, produces ligands with distinct developmental functions and receptor preferences.J Biol Chem. 2017 Nov 24;292(47):19160-19178. doi: 10.1074/jbc.M117.793513. Epub 2017 Sep 18. J Biol Chem. 2017. PMID: 28924042 Free PMC article.
-
Crimpy enables discrimination of presynaptic and postsynaptic pools of a BMP at the Drosophila neuromuscular junction.Dev Cell. 2014 Dec 8;31(5):586-98. doi: 10.1016/j.devcel.2014.10.006. Epub 2014 Nov 20. Dev Cell. 2014. PMID: 25453556 Free PMC article.
-
Bone morphogenetic protein signaling: the pathway and its regulation.Genetics. 2024 Feb 7;226(2):iyad200. doi: 10.1093/genetics/iyad200. Genetics. 2024. PMID: 38124338 Free PMC article. Review.
-
Spatial regulation of BMP activity.FEBS Lett. 2012 Jul 4;586(14):1929-41. doi: 10.1016/j.febslet.2012.02.035. Epub 2012 Feb 28. FEBS Lett. 2012. PMID: 22710177 Review.
Cited by
-
The Drosophila melanogaster Metabolic Response against Parasitic Nematode Infection Is Mediated by TGF-β Signaling.Microorganisms. 2020 Jun 29;8(7):971. doi: 10.3390/microorganisms8070971. Microorganisms. 2020. PMID: 32610560 Free PMC article.
-
Sterol regulatory element binding protein 1 couples mechanical cues and lipid metabolism.Nat Commun. 2019 Mar 22;10(1):1326. doi: 10.1038/s41467-019-09152-7. Nat Commun. 2019. PMID: 30902980 Free PMC article.
-
Fine-tuned shuttles for bone morphogenetic proteins.Curr Opin Genet Dev. 2013 Aug;23(4):374-84. doi: 10.1016/j.gde.2013.04.012. Epub 2013 Jun 2. Curr Opin Genet Dev. 2013. PMID: 23735641 Free PMC article. Review.
-
High-throughput screens using photo-highlighting discover BMP signaling in mitochondrial lipid oxidation.Nat Commun. 2017 Oct 11;8(1):865. doi: 10.1038/s41467-017-00944-3. Nat Commun. 2017. PMID: 29021566 Free PMC article.
-
Starvation-induced regulation of carbohydrate transport at the blood-brain barrier is TGF-β-signaling dependent.Elife. 2021 May 25;10:e62503. doi: 10.7554/eLife.62503. Elife. 2021. PMID: 34032568 Free PMC article.
References
-
- Arrese EL, Canavoso LE, Jouni ZE, Pennington JE, Tsuchida K, Wells MA. Lipid storage and mobilization in insects: current status and future directions. Insect Biochem Mol Biol. 2001;31:7–17. - PubMed
-
- Baggio B. Fatty acids, calcium and bone metabolism. J Nephrol. 2002;15:601–4. - PubMed
-
- Beenakkers AM, Van der Horst DJ, Van Marrewijk WJ. Insect lipids and lipoproteins, and their role in physiological processes. Prog Lipid Res. 1985;24:19–67. - PubMed
-
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. - PubMed
-
- Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

