Experimentally derived metastasis gene expression profile predicts recurrence and death in patients with colon cancer
- PMID: 19914252
- PMCID: PMC3388775
- DOI: 10.1053/j.gastro.2009.11.005
Experimentally derived metastasis gene expression profile predicts recurrence and death in patients with colon cancer
Abstract
Background & aims: Staging inadequately predicts metastatic risk in patients with colon cancer. We used a gene expression profile derived from invasive, murine colon cancer cells that were highly metastatic in an immunocompetent mouse model to identify patients with colon cancer at risk of recurrence.
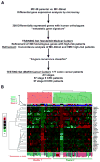
Methods: This phase 1, exploratory biomarker study used 55 patients with colorectal cancer from Vanderbilt Medical Center (VMC) as the training dataset and 177 patients from the Moffitt Cancer Center as the independent dataset. The metastasis-associated gene expression profile developed from the mouse model was refined with comparative functional genomics in the VMC gene expression profiles to identify a 34-gene classifier associated with high risk of metastasis and death from colon cancer. A metastasis score derived from the biologically based classifier was tested in the Moffitt dataset.
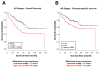
Results: A high score was significantly associated with increased risk of metastasis and death from colon cancer across all pathologic stages and specifically in stage II and stage III patients. The metastasis score was shown to independently predict risk of cancer recurrence and death in univariate and multivariate models. For example, among stage III patients, a high score translated to increased relative risk of cancer recurrence (hazard ratio, 4.7; 95% confidence interval, 1.566-14.05). Furthermore, the metastasis score identified patients with stage III disease whose 5-year recurrence-free survival was >88% and for whom adjuvant chemotherapy did not increase survival time.
Conclusion: A gene expression profile identified from an experimental model of colon cancer metastasis predicted cancer recurrence and death, independently of conventional measures, in patients with colon cancer.
Copyright 2010 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors disclose no conflicts.
Figures


References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Ragnhammar P, Hafstrom L, Nygren P, Glimelius B. A systematic overview of chemotherapy effects in colorectal cancer. Acta Oncol. 2001;40:282–308. - PubMed
-
- Benson AB, III, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–3419. - PubMed
-
- Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22:1797–1806. - PubMed
-
- Mamounas E, Wieand S, Wolmark N, et al. Comparative efficacy of adjuvant chemotherapy in patients with Dukes’ B versus Dukes’ C colon cancer: results from four National Surgical Adjuvant Breast and Bowel Project adjuvant studies (C-01, C-02, C-03, and C-04) J Clin Oncol. 1999;17:1349–1355. - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
- CA46413/CA/NCI NIH HHS/United States
- CA077839/CA/NCI NIH HHS/United States
- P50 CA095103/CA/NCI NIH HHS/United States
- R01 CA046413/CA/NCI NIH HHS/United States
- T32 CA106183/CA/NCI NIH HHS/United States
- U24 CA126588/CA/NCI NIH HHS/United States
- P01 CA077839/CA/NCI NIH HHS/United States
- CA106183/CA/NCI NIH HHS/United States
- CA084239/CA/NCI NIH HHS/United States
- U01 CA084239/CA/NCI NIH HHS/United States
- CA128323/CA/NCI NIH HHS/United States
- CA068485/CA/NCI NIH HHS/United States
- CA69457/CA/NCI NIH HHS/United States
- P50 CA128323/CA/NCI NIH HHS/United States
- DK52334/DK/NIDDK NIH HHS/United States
- CA112215/CA/NCI NIH HHS/United States
- R01 CA112215/CA/NCI NIH HHS/United States
- CA126588/CA/NCI NIH HHS/United States
- TL1 RR024978/RR/NCRR NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- R01 CA069457/CA/NCI NIH HHS/United States
- CA95103/CA/NCI NIH HHS/United States
- R01 DK052334/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

