Cardiac overexpression of metallothionein rescues cardiac contractile dysfunction and endoplasmic reticulum stress but not autophagy in sepsis
- PMID: 19914257
- PMCID: PMC2813369
- DOI: 10.1016/j.yjmcc.2009.11.003
Cardiac overexpression of metallothionein rescues cardiac contractile dysfunction and endoplasmic reticulum stress but not autophagy in sepsis
Abstract
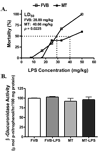
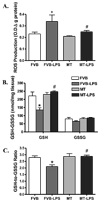
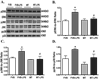
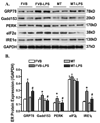
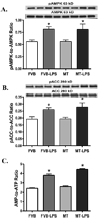
Sepsis is characterized by systematic inflammation where oxidative damage plays a key role in organ failure. This study was designed to examine the impact of the antioxidant metallothionein (MT) on lipopolysaccharide (LPS)-induced cardiac contractile and intracellular Ca(2+) dysfunction, oxidative stress, endoplasmic reticulum (ER) stress and autophagy. Mechanical and intracellular Ca(2+) properties were examined in hearts from FVB and cardiac-specific MT overexpression mice treated with LPS. Oxidative stress, activation of mitogen-activated protein kinase pathways (ERK, JNK and p38), ER stress, autophagy and inflammatory markers iNOS and TNFalpha were evaluated. Our data revealed enlarged end systolic diameter, decreased fractional shortening, myocyte peak shortening and maximal velocity of shortening/relengthening as well as prolonged duration of relengthening in LPS-treated FVB mice associated with reduced intracellular Ca(2+) release and decay. LPS treatment promoted oxidative stress (reduced glutathione/glutathione disulfide ratio and ROS generation). Western blot analysis revealed greater iNOS and TNFalpha, activation of ERK, JNK and p38, upregulation of ER stress markers GRP78, Gadd153, PERK and IRE1alpha, as well as the autophagy markers Beclin-1, LCB3 and Atg7 in LPS-treated mouse hearts without any change in total ERK, JNK and p38. Interestingly, these LPS-induced changes in echocardiographic, cardiomyocyte mechanical and intracellular Ca(2+) properties, ROS, stress signaling and ER stress (but not autophagy, iNOS and TNFalpha) were ablated by MT. Antioxidant N-acetylcysteine and the ER stress inhibitor tauroursodeoxycholic acid reversed LPS-elicited depression in cardiomyocyte contractile function. LPS activated AMPK and its downstream signaling ACC in conjunction with an elevated AMP/ATP ratio, which was unaffected by MT. Taken together, our data favor a beneficial effect of MT in the management of cardiac dysfunction in sepsis.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
Beneficial impact of cardiac heavy metal scavenger metallothionein in sepsis-provoked cardiac anomalies dependent upon regulation of endoplasmic reticulum stress and ferroptosis but not autophagy.Life Sci. 2024 Jan 1;336:122291. doi: 10.1016/j.lfs.2023.122291. Epub 2023 Nov 27. Life Sci. 2024. PMID: 38030060
-
Cardiac-specific overexpression of insulin-like growth factor I (IGF-1) rescues lipopolysaccharide-induced cardiac dysfunction and activation of stress signaling in murine cardiomyocytes.Shock. 2009 Jul;32(1):100-7. doi: 10.1097/SHK.0b013e31818ec609. Shock. 2009. PMID: 18948844 Free PMC article.
-
[Cardiac metallothionein overexpression improves cardiac contractile function and attenuates oxidative stress in lipopolysaccharide-treated mice].Zhonghua Xin Xue Guan Bing Za Zhi. 2011 Aug;39(8):711-6. Zhonghua Xin Xue Guan Bing Za Zhi. 2011. PMID: 22169416 Chinese.
-
Mechanisms of sepsis-induced cardiac dysfunction.Crit Care Med. 2007 Jun;35(6):1599-608. doi: 10.1097/01.CCM.0000266683.64081.02. Crit Care Med. 2007. PMID: 17452940 Review.
-
Redox signaling and cardiac sarcomeres.J Biol Chem. 2011 Mar 25;286(12):9921-7. doi: 10.1074/jbc.R110.175489. Epub 2011 Jan 21. J Biol Chem. 2011. PMID: 21257753 Free PMC article. Review.
Cited by
-
Chronic Akt activation attenuated lipopolysaccharide-induced cardiac dysfunction via Akt/GSK3β-dependent inhibition of apoptosis and ER stress.Biochim Biophys Acta. 2013 Jun;1832(6):848-63. doi: 10.1016/j.bbadis.2013.02.023. Epub 2013 Mar 6. Biochim Biophys Acta. 2013. PMID: 23474308 Free PMC article.
-
Mitochondria-endoplasmic reticulum contacts in sepsis-induced myocardial dysfunction.Front Cell Dev Biol. 2022 Nov 24;10:1036225. doi: 10.3389/fcell.2022.1036225. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36506093 Free PMC article. Review.
-
P27 Protects Cardiomyocytes from Sepsis via Activation of Autophagy and Inhibition of Apoptosis.Med Sci Monit. 2018 Nov 27;24:8565-8576. doi: 10.12659/MSM.912750. Med Sci Monit. 2018. PMID: 30478251 Free PMC article.
-
Hesperetin attenuates LPS-induced the inflammatory response and apoptosis of H9c2 by activating the AMPK/P53 signaling pathway.Immun Inflamm Dis. 2023 Aug;11(8):e973. doi: 10.1002/iid3.973. Immun Inflamm Dis. 2023. PMID: 37584301 Free PMC article.
-
Ablation of Akt2 protects against lipopolysaccharide-induced cardiac dysfunction: role of Akt ubiquitination E3 ligase TRAF6.J Mol Cell Cardiol. 2014 Sep;74:76-87. doi: 10.1016/j.yjmcc.2014.04.020. Epub 2014 May 5. J Mol Cell Cardiol. 2014. PMID: 24805195 Free PMC article.
References
-
- Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111–124. - PubMed
-
- The outcome of patients with sepsis and septic shock presenting to emergency departments in Australia and New Zealand. Crit Care Resusc. 2007;9(1):8–18. - PubMed
-
- Ren J, Wu S. A burning issue: do sepsis and systemic inflammatory response syndrome (SIRS) directly contribute to cardiac dysfunction? Front Biosci. 2006;11:15–22. - PubMed
-
- Bradford SD, Hunter K, Wu Y, Jablonowski C, Bahl JJ, Larson DF. Modulation of the inflammatory response in the cardiomyocyte and macrophage. J Extra Corpor Technol. 2001;33(3):167–174. - PubMed
-
- Niederbichler AD, Westfall MV, Su GL, Donnerberg J, Usman A, Vogt PM, et al. Cardiomyocyte function after burn injury and lipopolysaccharide exposure: single-cell contraction analysis and cytokine secretion profile. Shock. 2006;25(2):176–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

