Involvement of stromal p53 in tumor-stroma interactions
- PMID: 19914385
- PMCID: PMC2823948
- DOI: 10.1016/j.semcdb.2009.11.006
Involvement of stromal p53 in tumor-stroma interactions
Abstract
p53 is a major tumor-suppressor gene, inactivated by mutations in about half of all human cancer cases, and probably incapacitated by other means in most other cases. Most research regarding the role of p53 in cancer has focused on its ability to elicit apoptosis or growth arrest of cells that are prone to become malignant owing to DNA damage or oncogene activation, i.e. cell-autonomous activities of p53. However, p53 activation within a cell can also exert a variety of effects upon neighboring cells, through secreted factors and paracrine and endocrine mechanisms. Of note, p53 within cancer stromal cells can inhibit tumor growth and malignant progression. Cancer cells that evolve under this inhibitory influence acquire mechanisms to silence stromal p53, either by direct inhibition of p53 within stromal cells, or through pressure for selection of stromal cells with compromised p53 function. Hence, activation of stromal p53 by chemotherapy or radiotherapy might be part of the mechanisms by which these treatments cause cancer regression. However, in certain circumstances, activation of stromal p53 by cytotoxic anti-cancer agents might actually promote treatment resistance, probably through stromal p53-mediated growth arrest of the cancer cells or through protection of the tumor vasculature. Better understanding of the underlying molecular mechanisms is thus required. Hopefully, this will allow their manipulation towards better inhibition of cancer initiation, progression and metastasis.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures
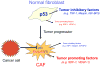
References
-
- Soussi T, Wiman KG. Shaping genetic alterations in human cancer: the p53 mutation paradigm. Cancer Cell. 2007;12:303–312. - PubMed
-
- Michael D, Oren M. The p53-Mdm2 module and the ubiquitin system. Semin Cancer Biol. 2003;13:49–58. - PubMed
-
- Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–431. - PubMed
-
- Menendez D, Inga A, Resnick MA. The expanding universe of p53 targets. Nat Rev Cancer. 2009;9:724–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

