Brain region specific actions of regulator of G protein signaling 4 oppose morphine reward and dependence but promote analgesia
- PMID: 19914603
- PMCID: PMC3077672
- DOI: 10.1016/j.biopsych.2009.08.041
Brain region specific actions of regulator of G protein signaling 4 oppose morphine reward and dependence but promote analgesia
Abstract
Background: Regulator of G protein signaling 4 (RGS4) is one of the smaller members of the RGS family of proteins, which are known to control signaling amplitude and duration via interactions with G protein alpha subunits or other signaling molecules. Earlier evidence suggests dynamic regulation of RGS4 levels in neuronal networks mediating actions of opiates and other drugs of abuse, but the consequences of RGS4 actions in vivo are largely unknown.
Methods: In this study, we use constitutive and nucleus accumbens-inducible RGS4 knockout mice as well as mice overexpressing RGS4 in the nucleus accumbens via viral mediated gene transfer, to examine the influence of RGS4 on behavioral responses to opiates. We also use electrophysiology and immunoprecipitation assays to further understand the mechanisms underlying the tissue-specific actions of RGS4.
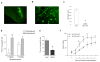
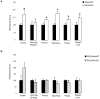
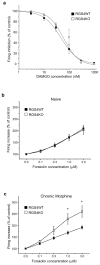
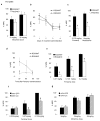
Results: Inducible knockout or selective overexpression of RGS4 in the nucleus accumbens reveals that, in this brain region, RGS4 acts as a negative regulator of morphine reward, whereas in the locus coeruleus RGS4 opposes morphine physical dependence. In contrast, we show that RGS4 does not affect morphine analgesia or tolerance but is a positive modulator of certain opiate analgesics, such as methadone and fentanyl.
Conclusions: These findings provide fundamentally novel information concerning the role of RGS4 in the cellular mechanisms underlying the diverse actions of opiate drugs in the nervous system.
Copyright 2010 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors reported no biomedical financial interests or potential conflicts of interest.
Figures
Similar articles
-
Regulator of G-Protein Signaling 4 (RGS4) Controls Morphine Reward by Glutamate Receptor Activation in the Nucleus Accumbens of Mouse Brain.Mol Cells. 2018 May 31;41(5):454-464. doi: 10.14348/molcells.2018.0023. Epub 2018 May 10. Mol Cells. 2018. PMID: 29754475 Free PMC article.
-
Regulator of G-Protein Signaling 7 Regulates Reward Behavior by Controlling Opioid Signaling in the Striatum.Biol Psychiatry. 2016 Aug 1;80(3):235-45. doi: 10.1016/j.biopsych.2015.07.026. Epub 2015 Aug 14. Biol Psychiatry. 2016. PMID: 26364547 Free PMC article.
-
Nucleus accumbens-specific interventions in RGS9-2 activity modulate responses to morphine.Neuropsychopharmacology. 2014 Jul;39(8):1968-77. doi: 10.1038/npp.2014.45. Epub 2014 Feb 24. Neuropsychopharmacology. 2014. PMID: 24561386 Free PMC article.
-
Reflections on: "A general role for adaptations in G-Proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function".Brain Res. 2016 Aug 15;1645:71-4. doi: 10.1016/j.brainres.2015.12.039. Epub 2015 Dec 29. Brain Res. 2016. PMID: 26740398 Free PMC article. Review.
-
A role of RGS proteins in drug addiction.Biochem Pharmacol. 2008 Jan 1;75(1):76-84. doi: 10.1016/j.bcp.2007.07.045. Epub 2007 Aug 11. Biochem Pharmacol. 2008. PMID: 17880927 Review.
Cited by
-
Identification of a Novel Population of Neuromedin S Expressing Neurons in the Ventral Tegmental Area That Promote Morphine-Elicited Behavior.J Neurosci. 2025 Mar 26;45(13):e1662242025. doi: 10.1523/JNEUROSCI.1662-24.2025. J Neurosci. 2025. PMID: 39929726
-
The role of regulator of G protein signaling 4 in delta-opioid receptor-mediated behaviors.Psychopharmacology (Berl). 2017 Jan;234(1):29-39. doi: 10.1007/s00213-016-4432-5. Epub 2016 Sep 13. Psychopharmacology (Berl). 2017. PMID: 27624599 Free PMC article.
-
RGS4 Maintains Chronic Pain Symptoms in Rodent Models.J Neurosci. 2019 Oct 16;39(42):8291-8304. doi: 10.1523/JNEUROSCI.3154-18.2019. Epub 2019 Jul 15. J Neurosci. 2019. PMID: 31308097 Free PMC article.
-
The brain reward circuitry in mood disorders.Nat Rev Neurosci. 2013 Sep;14(9):609-25. doi: 10.1038/nrn3381. Epub 2013 Aug 14. Nat Rev Neurosci. 2013. PMID: 23942470 Free PMC article. Review.
-
Reduced striatal M4-cholinergic signaling following dopamine loss contributes to parkinsonian and l-DOPA-induced dyskinetic behaviors.Sci Adv. 2024 Nov 22;10(47):eadp6301. doi: 10.1126/sciadv.adp6301. Epub 2024 Nov 20. Sci Adv. 2024. PMID: 39565858 Free PMC article.
References
-
- Dohlman HG, Thorner J. RGS proteins and signaling by heterotrimeric G proteins. J Biol Chem. 1997;272:3871–3874. - PubMed
-
- Berman DM, Gilman AG. Mammalian RGS proteins: barbarians at the gates. J Bio Chem. 1998;273:1269–1272. - PubMed
-
- Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev. 2002;54:527–559. - PubMed
-
- Terzi D, Stergiou E, King SL, Zachariou V. Regulators of G protein signaling and neuropsychiatric disorders. Progress in Molecular Biology and Translational Science. 2009;86 in press. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

