BAFF overexpression promotes anti-dsDNA B-cell maturation and antibody secretion
- PMID: 19914608
- PMCID: PMC2812582
- DOI: 10.1016/j.cellimm.2009.10.004
BAFF overexpression promotes anti-dsDNA B-cell maturation and antibody secretion
Abstract
Overexpression of BAFF is believed to play an important role in systemic lupus erythematosus and elevated levels of serum BAFF have been found in lupus patients. Excess BAFF also leads to overproduction of anti-dsDNA antibodies and a lupus-like syndrome in mice. In the present study, we use mice transgenic for the R4A-Cmu (IgM) heavy chain of an anti-dsDNA antibody, to study the effects of BAFF overexpression on anti-dsDNA B-cell regulation. We observe that overexpression of BAFF promotes anti-dsDNA B-cell maturation and secretion of antibody and enriches for transgenic anti-dsDNA B cells in the marginal zone and follicular splenic compartments. In addition, our data suggests that BAFF rescues a subset of anti-dsDNA B cells from a regulatory checkpoint in the transitional stage of development.
2009 Elsevier Inc. All rights reserved.
Conflict of interest statement
There are no commercial or financial conflicts of interest associated with this study.
Figures
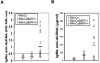

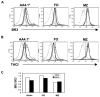







Similar articles
-
CAR-T cell targeting three receptors on autoreactive B cells for systemic lupus erythematosus therapy.J Autoimmun. 2025 Feb;151:103369. doi: 10.1016/j.jaut.2025.103369. Epub 2025 Jan 19. J Autoimmun. 2025. PMID: 39832454
-
Expression of B-cell activating factor of the tumour necrosis factor family (BAFF) in T cells in active systemic lupus erythematosus: the role of BAFF in T cell-dependent B cell pathogenic autoantibody production.Rheumatology (Oxford). 2007 Jul;46(7):1083-6. doi: 10.1093/rheumatology/kem097. Epub 2007 May 11. Rheumatology (Oxford). 2007. PMID: 17500077
-
Development of autoimmune nephritis in genetically asplenic and splenectomized BAFF transgenic mice.J Autoimmun. 2011 Mar;36(2):125-34. doi: 10.1016/j.jaut.2010.12.002. Epub 2011 Jan 7. J Autoimmun. 2011. PMID: 21216131
-
Does the BAFF dysregulation play a major role in the pathogenesis of systemic lupus erythematosus?J Autoimmun. 2008 Feb-Mar;30(1-2):63-7. doi: 10.1016/j.jaut.2007.11.001. Epub 2007 Dec 21. J Autoimmun. 2008. PMID: 18155417 Review.
-
BAFF and innate immunity: new therapeutic targets for systemic lupus erythematosus.Immunol Cell Biol. 2012 Mar;90(3):293-303. doi: 10.1038/icb.2011.111. Epub 2012 Jan 10. Immunol Cell Biol. 2012. PMID: 22231653 Review.
Cited by
-
Transgenic overexpression of BAFF regulates the expression of immune-related genes in zebrafish, Danio rerio.J Genet. 2016 Dec;95(4):751-760. doi: 10.1007/s12041-016-0690-6. J Genet. 2016. PMID: 27994173
-
Lupus and recurrent pregnancy loss: the role of female sex hormones and B cells.Front Endocrinol (Lausanne). 2023 Oct 3;14:1233883. doi: 10.3389/fendo.2023.1233883. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37859991 Free PMC article. Review.
-
Elevated serum levels of B-cell activating factor in pediatric renal transplant patients.Pediatr Nephrol. 2012 Aug;27(8):1389-95. doi: 10.1007/s00467-012-2142-8. Epub 2012 Mar 28. Pediatr Nephrol. 2012. PMID: 22453734
-
Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps.J Allergy Clin Immunol. 2011 Dec;128(6):1198-1206.e1. doi: 10.1016/j.jaci.2011.08.037. Epub 2011 Oct 13. J Allergy Clin Immunol. 2011. PMID: 21996343 Free PMC article.
-
The role of B cell-activating factor system in autoimmune diseases: mechanisms, disease implications, and therapeutic advances.Front Immunol. 2025 Jun 6;16:1538555. doi: 10.3389/fimmu.2025.1538555. eCollection 2025. Front Immunol. 2025. PMID: 40547016 Free PMC article. Review.
References
-
- Rolink AG, Tschopp J, Schneider P, Melchers F. BAFF is a survival and maturation factor for mouse B cells. Eur J Immunol. 2002;32:2004–2010. - PubMed
-
- Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew e, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. - PubMed
-
- Schneider P, Takatsuka H, Wilson A, Mackay F, Tardivel A, Lens S, Cachero TG, Finke D, Beermann F, Tschopp J. Maturation of Marginal zone and Follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J Exp Med. 2001;194:1691–1697. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

