Signaling mechanisms of the Mycobacterium tuberculosis receptor Ser/Thr protein kinases
- PMID: 19914822
- PMCID: PMC2790423
- DOI: 10.1016/j.sbi.2009.10.017
Signaling mechanisms of the Mycobacterium tuberculosis receptor Ser/Thr protein kinases
Abstract
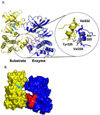
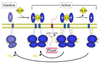
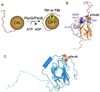
Like eukaryotes, bacteria express receptor Ser/Thr protein kinases (STPKs) that initiate a wide variety of signaling networks. Recent biochemical and structural studies of the STPKs of Mycobacterium tuberculosis have revealed that bacterial and eukaryotic STPKs adopt common folds and share mechanisms of substrate recognition and regulation. Mycobacterial receptor STPKs are activated by dimerization through two distinct interfaces that promote activation-loop phosphorylation. The active STPKs phosphorylate diverse substrates within the bacterial cell, including other kinases as well as proteins involved in many central physiological processes. In the case of the FHA-domain protein, GarA, the unphosphorylated protein regulates primary metabolism, while phosphorylation mediates GarA autoinhibition. These studies have begun to define the activation mechanisms and the biological regulatory functions of the mycobacterial STPKs.
Figures

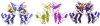
References
-
-
Mougous JD, Gifford CA, Ramsdell TL, Mekalanos JJ. Threonine phosphorylation post-translationally regulates protein secretion in Pseudomonas aeruginosa. Nat Cell Biol. 2007;9:797–803. Genetic and biochemical analysis showed the central roles of the PpkA STPK, the antagonistic PppA Ser/Thr phosphatase and the Fha1 FHA-domain protein in assembling the type VI secretion apparatus in P. aeruginosa.
-
-
-
Hsu F, Schwarz S, Mougous JD. TagR promotes PpkA-catalysed type VI secretion activation in Pseudomonas aeruginosa. Mol Microbiol. 2009;72:1111–1125. A fascinating study of type VI secretion in P. aeruginosa established a cascade of assembly reactions triggered by activation of the PpkA STPK by dimerization in vivo.
-
-
-
S Shah IM, Laaberki MH, Popham DL, Dworkin J. A eukaryotic-like Ser/Thr kinase signals bacteria to exit dormancy in response to peptidoglycan fragments. Cell. 2008;135:486–496. A beautiful demonstration that peptidoglycan fragments signal through PknB orthologs, and the species specificity of signaling is established by the correspondence between the extracellular PASTA domains of the STPK and chemical variations in peptidoglycan.
-
-
-
Av-Gay Y, Everett M. The eukaryotic-like Ser/Thr protein kinases of Mycobacterium tuberculosis. Trends Microbiol. 2000;8:238–244. The classic review of the Mtb kinome.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

