A nonadjuvanted polypeptide nanoparticle vaccine confers long-lasting protection against rodent malaria
- PMID: 19915055
- PMCID: PMC4528972
- DOI: 10.4049/jimmunol.0901957
A nonadjuvanted polypeptide nanoparticle vaccine confers long-lasting protection against rodent malaria
Abstract
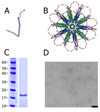
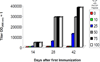
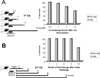
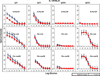
We have designed and produced a prototypic malaria vaccine based on a highly versatile self-assembling polypeptide nanoparticle (SAPN) platform that can repetitively display antigenic epitopes. We used this platform to display a tandem repeat of the B cell immunodominant repeat epitope (DPPPPNPN)(2)D of the malaria parasite Plasmodium berghei circumsporozoite protein. Administered in saline, without the need for a heterologous adjuvant, the SAPN construct P4c-Mal conferred a long-lived, protective immune response to mice with a broad range of genetically distinct immune backgrounds including the H-2(b), H-2(d), and H-2(k) alleles. Immunized mice produced a CD4(+) T cell-dependent, high-titer, long-lasting, high-avidity Ab response against the B cell epitope. Mice were protected against an initial challenge of parasites up to 6 mo after the last immunization or for up to 15 mo against a second challenge after an initial challenge of parasites had successfully been cleared. Furthermore, we demonstrate that the SAPN platform not only functions to deliver an ordered repetitive array of B cell peptide epitopes but operates as a classical immunological carrier to provide cognate help to the P4c-Mal-specific B cells.
Figures




Similar articles
-
Protective antibody and CD8+ T-cell responses to the Plasmodium falciparum circumsporozoite protein induced by a nanoparticle vaccine.PLoS One. 2012;7(10):e48304. doi: 10.1371/journal.pone.0048304. Epub 2012 Oct 29. PLoS One. 2012. PMID: 23144750 Free PMC article.
-
Linear and multiple antigen peptides containing defined T and B epitopes of the Plasmodium yoelii circumsporozoite protein: antibody-mediated protection and boosting by sporozoite infection.Int Immunol. 1997 Dec;9(12):1817-24. doi: 10.1093/intimm/9.12.1817. Int Immunol. 1997. PMID: 9466309
-
Re-investigation of the circumsporozoite protein-based induction of sterile immunity against Plasmodium berghei infection.Vaccine. 1996 Jun;14(8):828-36. doi: 10.1016/0264-410x(95)00175-z. Vaccine. 1996. PMID: 8817831
-
Immune responses to liver-stage parasites: implications for vaccine development.Chem Immunol. 2002;80:97-124. doi: 10.1159/000058841. Chem Immunol. 2002. PMID: 12058653 Review. No abstract available.
-
Transgenic rodent Plasmodium berghei parasites as tools for assessment of functional immunogenicity and optimization of human malaria vaccines.Eukaryot Cell. 2008 Nov;7(11):1875-9. doi: 10.1128/EC.00242-08. Epub 2008 Sep 19. Eukaryot Cell. 2008. PMID: 18806208 Free PMC article. Review. No abstract available.
Cited by
-
Nanoparticles Carrying Conserved Regions of Influenza A Hemagglutinin, Nucleoprotein, and M2 Protein Elicit a Strong Humoral and T Cell Immune Response and Protect Animals from Infection.Molecules. 2023 Sep 5;28(18):6441. doi: 10.3390/molecules28186441. Molecules. 2023. PMID: 37764217 Free PMC article.
-
Head-to-Head Comparison of Soluble vs. Qβ VLP Circumsporozoite Protein Vaccines Reveals Selective Enhancement of NANP Repeat Responses.PLoS One. 2015 Nov 16;10(11):e0142035. doi: 10.1371/journal.pone.0142035. eCollection 2015. PLoS One. 2015. PMID: 26571021 Free PMC article.
-
Self-Assembling Peptide Epitopes as Novel Platform for Anticancer Vaccination.Mol Pharm. 2017 May 1;14(5):1482-1493. doi: 10.1021/acs.molpharmaceut.6b01003. Epub 2017 Jan 27. Mol Pharm. 2017. PMID: 28088862 Free PMC article.
-
Optimized Refolding Buffers Oriented Humoral Immune Responses Versus PfGCS1 Self-Assembled Peptide Nanoparticle.Mol Biotechnol. 2024 Sep;66(9):2648-2664. doi: 10.1007/s12033-023-01044-y. Epub 2024 Jan 24. Mol Biotechnol. 2024. PMID: 38267696
-
Plasmodium falciparum synthetic LbL microparticle vaccine elicits protective neutralizing antibody and parasite-specific cellular immune responses.Vaccine. 2013 Apr 8;31(15):1898-904. doi: 10.1016/j.vaccine.2013.02.027. Epub 2013 Feb 26. Vaccine. 2013. PMID: 23481177 Free PMC article.
References
-
- Ballou WR, Arevalo-Herrera M, Carucci D, Richie TL, Corradin G, Diggs C, Druilhe P, Giersing BK, Saul A, Heppner DG, Kester KE, Lanar DE, Lyon J, Hill AV, Pan W, Cohen JD. Update on the clinical development of candidate malaria vaccines. The American journal of tropical medicine and hygiene. 2004;71:239–247. - PubMed
-
- Epstein JE, Giersing B, Mullen G, Moorthy V, Richie TL. Malaria vaccines: are we getting closer? Current opinion in molecular therapeutics. 2007;9:12–24. - PubMed
-
- Bejon P, Mwacharo J, Kai O, Mwangi T, Milligan P, Todryk S, Keating S, Lang T, Lowe B, Gikonyo C, Molyneux C, Fegan G, Gilbert SC, Peshu N, Marsh K, Hill AV. A phase 2b randomised trial of the candidate malaria vaccines FP9 ME-TRAP and MVA ME-TRAP among children in Kenya. PLoS clinical trials. 2006;1:e29. - PMC - PubMed
-
- Dunachie SJ, Walther M, Vuola JM, Webster DP, Keating SM, Berthoud T, Andrews L, Bejon P, Poulton I, Butcher G, Watkins K, Sinden RE, Leach A, Moris P, Tornieporth N, Schneider J, Dubovsky F, Tierney E, Williams J, Heppner DG, Jr, Gilbert SC, Cohen J, Hill AV. A clinical trial of prime-boost immunisation with the candidate malaria vaccines RTS,S/AS02A and MVA-CS. Vaccine. 2006;24:2850–2859. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

