In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells
- PMID: 19915570
- PMCID: PMC3663137
- DOI: 10.1038/nnano.2009.333
In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells
Abstract
The spread of cancer cells between organs, a process known as metastasis, is the cause of most cancer deaths. Detecting circulating tumour cells -- a common marker for the development of metastasis -- is difficult because ex vivo methods are not sensitive enough owing to limited blood sample volume and in vivo diagnosis is time-consuming as large volumes of blood must be analysed. Here, we show a way to magnetically capture circulating tumour cells in the bloodstream of mice followed by rapid photoacoustic detection. Magnetic nanoparticles, which were functionalized to target a receptor commonly found in breast cancer cells, bound and captured circulating tumour cells under a magnet. To improve detection sensitivity and specificity, gold-plated carbon nanotubes conjugated with folic acid were used as a second contrast agent for photoacoustic imaging. By integrating in vivo multiplex targeting, magnetic enrichment, signal amplification and multicolour recognition, our approach allows circulating tumour cells to be concentrated from a large volume of blood in the vessels of tumour-bearing mice, and this could have potential for the early diagnosis of cancer and the prevention of metastasis in humans.
Figures

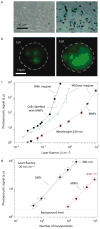


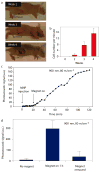
Comment in
-
Nanomedicine: detecting rare cancer cells.Nat Nanotechnol. 2009 Dec;4(12):798-9. doi: 10.1038/nnano.2009.367. Nat Nanotechnol. 2009. PMID: 19966826 No abstract available.
References
-
- Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. - PubMed
-
- Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nature Rev Cancer. 2008;8:329–340. - PubMed
-
- Riethdorf S, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13:920–928. - PubMed
-
- Georgakoudi I, et al. In vivo flow cytometry: a new method for enumerating circulating cancer cells. Cancer Res. 2004;64:5044–5047. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

