Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions
- PMID: 19915614
- PMCID: PMC2820596
- DOI: 10.1038/onc.2009.389
Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions
Abstract
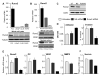
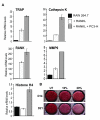
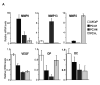
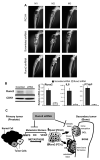
Runx2, a bone-specific transcriptional regulator, is abnormally expressed in highly metastatic prostate cancer cells. Here, we identified the functional activities of Runx2 in facilitating tumor growth and osteolysis. Our studies show that negligible Runx2 is found in normal prostate epithelial and non-metastatic LNCaP prostate cancer cells. In the intra-tibial metastasis model, high Runx2 levels are associated with development of large tumors, increased expression of metastasis-related genes (MMP9, MMP13, VEGF, Osteopontin) and secreted bone-resorbing factors (PTHrP, IL8) promoting osteolytic disease. Runx2 siRNA treatment of PC3 cells decreased cell migration and invasion through Matrigel in vitro, and in vivo shRunx2 expression in PC3 cells blocked their ability to survive in the bone microenvironment. Mechanisms of Runx2 function were identified in co-culture studies showing that PC3 cells promote osteoclastogenesis and inhibit osteoblast activity. The clinical significance of these findings is supported by human tissue microarray studies of prostate tumors at stages of cancer progression, in which Runx2 is expressed in both adenocarcinomas and metastatic tumors. Together these findings indicate that Runx2 is a key regulator of events associated with prostate cancer metastatic bone disease.
Figures


References
-
- Araki S, Omori Y, Lyn D, Singh RK, Meinbach DM, Sandman Y, et al. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 2007;67:6854–6862. - PubMed
-
- Armstrong AP, Miller RE, Jones JC, Zhang J, Keller ET, Dougall WC. RANKL acts directly on RANK-expressing prostate tumor cells and mediates migration and expression of tumor metastasis genes. Prostate. 2008;68:92–104. - PubMed
-
- Barnes GL, Hebert KE, Kamal M, Javed A, Einhorn TA, Lian JB, et al. Fidelity of Runx2 activity in breast cancer cells is required for the generation of metastases associated osteolytic disease. Cancer Res. 2004;64:4506–4513. - PubMed
-
- Barnes GL, Javed A, Waller SM, Kamal MH, Hebert KE, Hassan MQ, et al. Osteoblast-related transcription factors Runx2 (Cbfa1/AML3) and MSX2 mediate the expression of bone sialoprotein in human metastatic breast cancer cells. Cancer Res. 2003;63:2631–2637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01CA090917/CA/NCI NIH HHS/United States
- P01CA082834/CA/NCI NIH HHS/United States
- R01 AR039588/AR/NIAMS NIH HHS/United States
- R37 DE012528/DE/NIDCR NIH HHS/United States
- R01 AR049069/AR/NIAMS NIH HHS/United States
- P01 CA082834/CA/NCI NIH HHS/United States
- R01 CA069158/CA/NCI NIH HHS/United States
- R01 CA089720/CA/NCI NIH HHS/United States
- R01 CA109874/CA/NCI NIH HHS/United States
- R01CA069158/CA/NCI NIH HHS/United States
- P50CA69568/CA/NCI NIH HHS/United States
- P01CA093900/CA/NCI NIH HHS/United States
- R01 CA090917/CA/NCI NIH HHS/United States
- P50 CA069568/CA/NCI NIH HHS/United States
- R01CA89720/CA/NCI NIH HHS/United States
- P01 CA093900/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

