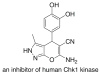
Organocatalyzed enantioselective synthesis of 6-amino-5-cyanodihydropyrano[2,3-c]pyrazoles
- PMID: 19915654
- PMCID: PMC2745181
- DOI: 10.1016/j.tetlet.2009.02.210
Organocatalyzed enantioselective synthesis of 6-amino-5-cyanodihydropyrano[2,3-c]pyrazoles
Abstract
The first enantioselective synthesis of biologically active 6-amino-5-cyanodihydropyrano[2,3-c]pyrazoles has been achieved through a cinchona alkaloid-catalyzed tandem Michael addition and Thorpe-Ziegler type reaction between 2-pyrazolin-5-ones and benzylidenemalononitriles. The reaction may also be carried out in a three-component or a four-component fashion via the in situ formation of these two components from simple and readily available starting materials. The desired products were obtained in excellent yields with mediocre to excellent enantioselectivities (up to >99% ee).
Figures
References
-
- Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Proc Natl Acad Sci USA. 2000;97:7124–7129. - PMC - PubMed
- El-Tamany ES, El-Shahed FA, Mohamed BH. J Serb Chem Soc. 1999;64:9–18.
- Zaki MEA, Soliman HA, Hiekal OA, Rashad AE. Z Naturforsch C. 2006;61:1–5. - PubMed
- Ismail ZH, Aly GM, El-Degwi MS, Heiba HI, Ghorab MM. Egypt J Biotech. 2003;13:73–82.
- Abdelrazek FM, Metz P, Metwally NH, El-Mahrouky SF. Arch Pharm. 2006;339:456–460. - PubMed
- Abdelrazek FM, Metz P, Kataeva O, Jaeger A, El-Mahrouky SF. Arch Pharm. 2007;340:543–548. - PubMed
- Foloppe N, Fisher LM, Howes R, Potter A, Robertson AGS, Surgenor AE. Bioorg Med Chem. 2006;14:4792–4802. - PubMed
-
- Otto HH. Arch Pharm. 1974;307:444–447. - PubMed
- Otto HH, Schmelz H. Arch Pharm. 1979;312:478–486.
-
- Sharanin YA, Sharanina LG, Puzanova VV. Zh Org Khim. 1983;19:2609–2615.
-
-
For examples, see: Lehmann F, Holm M, Laufer S. J Comb Chem. 2008;10:364–367. and references cited therein.
-
-
- Shestopalov AM, Yakubov AP, Tsyganov DV, Emel’yanova Yu M, Nesterov VN. Chem Heterocycl Compd. 2002;38:1180–1189.
Grants and funding
LinkOut - more resources
Full Text Sources