Arachidonic Acid Activates K-Cl-cotransport in HepG2 Human Hepatoblastoma Cells
- PMID: 19915704
- PMCID: PMC2776902
- DOI: 10.4196/kjpp.2009.13.5.401
Arachidonic Acid Activates K-Cl-cotransport in HepG2 Human Hepatoblastoma Cells
Abstract
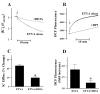
K(+)-Cl(-)-cotransport (KCC) has been reported to have various cellular functions, including proliferation and apoptosis of human cancer cells. However, the signal transduction pathways that control the activity of KCC are currently not well understood. In this study we investigated the possible role of phospholipase A(2) (PLA(2))-arachidonic acid (AA) signal in the regulatory mechanism of KCC activity. Exogenous application of AA significantly induced K(+) efflux in a dose-dependent manner, which was completely blocked by R-(+)-[2-n-butyl-6,7-dichloro-2-cyclopentyl-2,3-dihydro-1-oxo-1H-inden-5-yl]oxy]acetic acid (DIOA), a specific KCC inhibitor. N-Ethylmaleimide (NEM), a KCC activator-induced K(+) efflux was significantly suppressed by bromoenol lactone (BEL), an inhibitor of the calcium-independent PLA(2) (iPLA(2)), whereas it was not significantly altered by arachidonyl trifluoromethylketone (AACOCF(3)) and p-bromophenacyl bromide (BPB), inhibitors of the calcium-dependent cytosolic PLA(2) (cPLA(2)) and the secretory PLA(2) (sPLA(2)), respectively. NEM increased AA liberation in a dose- and time-dependent manner, which was markedly prevented only by BEL. In addition, the NEM-induced ROS generation was significantly reduced by DPI and BEL, whereas AACOCF(3) and BPB did not have an influence. The NEM-induced KCC activation and ROS production was not significantly affected by treatment with indomethacin (Indo) and nordihydroguaiaretic acid (NDGA), selective inhibitors of cyclooxygenase (COX) and lipoxygenase (LOX), respectively. Treatment with 5,8,11,14-eicosatetraynoic acid (ETYA), a non-metabolizable analogue of AA, markedly produced ROS and activated the KCC. Collectively, these results suggest that iPLA(2)-AA signal may be essentially involved in the mechanism of ROS-mediated KCC activation in HepG2 cells.
Keywords: Arachidonic acid; HepG2 cells; K+-Cl--cotransport; N-Ethylmaleimide; Phospholipase A2; Reactive oxygen species.
Figures
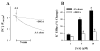

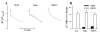

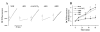
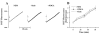
References
-
- Adragna NC, Di Fulvio M, Lauf PK. Regulation of K-Cl cotransport: from function to genes. J Membr Biol. 2004;201:109–137. - PubMed
-
- Adragna NC, Ferrell CM, Zhang J, Di Fulvio M, Temprana CF, Sharma A, Fyffe RE, Cool DR, Lauf PK. Signal transduction mechanisms of K+-Cl- cotransport regulation and relationship to disease. Acta Physiol (Oxf) 2006;187:125–139. - PubMed
-
- Adragna NC, White RE, Orlov SN, Lauf PK. K-Cl cotransport in vascular smooth muscle and erythrocytes: possible implication in vasodilation. Am J Physiol Cell Physiol. 2000;278:C381–C390. - PubMed
-
- Amlal H, Paillard M, Bichara M. Cl--dependent NH4+ transport mechanisms in medullary thick ascending limb cells. Am J Physiol. 1994;267:C1607–C1615. - PubMed
-
- Bakalova R, Matsura T, Kanno I. The cyclooxygenase inhibitors indomethacin and rofecoxib reduce regional cerebral blood flow evoked by somatosensory stimulation in rats. Exp Biol Med (Maywood) 2002;227:465–473. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

