Dermatitis and aging-related barrier dysfunction in transgenic mice overexpressing an epidermal-targeted claudin 6 tail deletion mutant
- PMID: 19915705
- PMCID: PMC2773045
- DOI: 10.1371/journal.pone.0007814
Dermatitis and aging-related barrier dysfunction in transgenic mice overexpressing an epidermal-targeted claudin 6 tail deletion mutant
Abstract
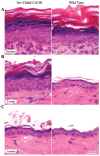
The barrier function of the skin protects the mammalian body against infection, dehydration, UV irradiation and temperature fluctuation. Barrier function is reduced with the skin's intrinsic aging process, however the molecular mechanisms involved are unknown. We previously demonstrated that Claudin (Cldn)-containing tight junctions (TJs) are essential in the development of the epidermis and that transgenic mice overexpressing Cldn6 in the suprabasal layers of the epidermis undergo a perturbed terminal differentiation program characterized in part by reduced barrier function. To dissect further the mechanisms by which Cldn6 acts during epithelial differentiation, we overexpressed a Cldn6 cytoplasmic tail deletion mutant in the suprabasal compartment of the transgenic mouse epidermis. Although there were no gross phenotypic abnormalities at birth, subtle epidermal anomalies were present that disappeared by one month of age, indicative of a robust injury response. However, with aging, epidermal changes with eventual chronic dermatitis appeared with a concomitant barrier dysfunction manifested in increased trans-epidermal water loss. Immunohistochemical analysis revealed aberrant suprabasal Cldn localization with marked down-regulation of Cldn1. Both the proliferative and terminal differentiation compartments were perturbed as evidenced by mislocalization of multiple epidermal markers. These results suggest that the normally robust injury response mechanism of the epidermis is lost in the aging Involucrin-Cldn6-CDelta196 transgenic epidermis, and provide a model for evaluation of aging-related skin changes.
Conflict of interest statement
Figures






References
-
- Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3:199–209. - PubMed
-
- Turksen K, Troy TC. Epidermal cell lineage. Biochem Cell Biol. 1998;76:889–898. - PubMed
-
- Pouillot A, Dayan N, Polla AS, Polla LL, Polla BS. The stratum corneum: a double paradox. J Cosmet Dermatol. 2008;7:143–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

