The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion
- PMID: 19915718
- PMCID: PMC2774229
- DOI: 10.1371/journal.ppat.1000660
The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion
Abstract
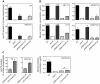
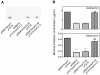
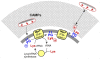
Many bacterial pathogens achieve resistance to defensin-like cationic antimicrobial peptides (CAMPs) by the multiple peptide resistance factor (MprF) protein. MprF plays a crucial role in Staphylococcus aureus virulence and it is involved in resistance to the CAMP-like antibiotic daptomycin. MprF is a large membrane protein that modifies the anionic phospholipid phosphatidylglycerol with l-lysine, thereby diminishing the bacterial affinity for CAMPs. Its widespread occurrence recommends MprF as a target for novel antimicrobials, although the mode of action of MprF has remained incompletely understood. We demonstrate that the hydrophilic C-terminal domain and six of the fourteen proposed trans-membrane segments of MprF are sufficient for full-level lysyl-phosphatidylglycerol (Lys-PG) production and that several conserved amino acid positions in MprF are indispensable for Lys-PG production. Notably, Lys-PG production did not lead to efficient CAMP resistance and most of the Lys-PG remained in the inner leaflet of the cytoplasmic membrane when the large N-terminal hydrophobic domain of MprF was absent, indicating a crucial role of this protein part. The N-terminal domain alone did not confer CAMP resistance or repulsion of the cationic test protein cytochrome c. However, when the N-terminal domain was coexpressed with the Lys-PG synthase domain either in one protein or as two separate proteins, full-level CAMP resistance was achieved. Moreover, only coexpression of the two domains led to efficient Lys-PG translocation to the outer leaflet of the membrane and to full-level cytochrome c repulsion, indicating that the N-terminal domain facilitates the flipping of Lys-PG. Thus, MprF represents a new class of lipid-biosynthetic enzymes with two separable functional domains that synthesize Lys-PG and facilitate Lys-PG translocation. Our study unravels crucial details on the molecular basis of an important bacterial immune evasion mechanism and it may help to employ MprF as a target for new anti-virulence drugs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Alanyl-phosphatidylglycerol and lysyl-phosphatidylglycerol are translocated by the same MprF flippases and have similar capacities to protect against the antibiotic daptomycin in Staphylococcus aureus.Antimicrob Agents Chemother. 2012 Jul;56(7):3492-7. doi: 10.1128/AAC.00370-12. Epub 2012 Apr 9. Antimicrob Agents Chemother. 2012. PMID: 22491694 Free PMC article.
-
The lipid-modifying multiple peptide resistance factor is an oligomer consisting of distinct interacting synthase and flippase subunits.mBio. 2015 Jan 27;6(1):e02340-14. doi: 10.1128/mBio.02340-14. mBio. 2015. PMID: 25626904 Free PMC article.
-
Broad-spectrum antimicrobial peptide resistance by MprF-mediated aminoacylation and flipping of phospholipids.Mol Microbiol. 2011 Apr;80(2):290-9. doi: 10.1111/j.1365-2958.2011.07576.x. Epub 2011 Mar 1. Mol Microbiol. 2011. PMID: 21306448 Review.
-
Multiple peptide resistance factor (MprF)-mediated Resistance of Staphylococcus aureus against antimicrobial peptides coincides with a modulated peptide interaction with artificial membranes comprising lysyl-phosphatidylglycerol.J Biol Chem. 2011 May 27;286(21):18692-700. doi: 10.1074/jbc.M111.226886. Epub 2011 Apr 7. J Biol Chem. 2011. PMID: 21474443 Free PMC article.
-
MprF-mediated daptomycin resistance.Int J Med Microbiol. 2019 Jul;309(5):359-363. doi: 10.1016/j.ijmm.2019.05.010. Epub 2019 Jun 4. Int J Med Microbiol. 2019. PMID: 31182276 Review.
Cited by
-
Resistance phenotypes mediated by aminoacyl-phosphatidylglycerol synthases.J Bacteriol. 2012 Mar;194(6):1401-16. doi: 10.1128/JB.06576-11. Epub 2012 Jan 20. J Bacteriol. 2012. PMID: 22267511 Free PMC article.
-
Vancomycin tolerance of adherent Staphylococcus aureus is impeded by nanospike-induced physiological changes.NPJ Biofilms Microbiomes. 2023 Nov 29;9(1):90. doi: 10.1038/s41522-023-00458-5. NPJ Biofilms Microbiomes. 2023. PMID: 38030708 Free PMC article.
-
Evolution of Enterococcus faecium in Response to a Combination of Daptomycin and Fosfomycin Reveals Distinct and Diverse Adaptive Strategies.Antimicrob Agents Chemother. 2022 Jun 21;66(6):e0233321. doi: 10.1128/aac.02333-21. Epub 2022 May 11. Antimicrob Agents Chemother. 2022. PMID: 35543524 Free PMC article.
-
Proteolytic cleavage inactivates the Staphylococcus aureus lipoteichoic acid synthase.J Bacteriol. 2011 Oct;193(19):5279-91. doi: 10.1128/JB.00369-11. Epub 2011 Jul 22. J Bacteriol. 2011. PMID: 21784926 Free PMC article.
-
Exposure of Methicillin-Resistant Staphylococcus aureus to Low Levels of the Antibacterial THAM-3ΦG Generates a Small Colony Drug-Resistant Phenotype.Sci Rep. 2018 Jun 29;8(1):9850. doi: 10.1038/s41598-018-28283-3. Sci Rep. 2018. PMID: 29959441 Free PMC article.
References
-
- Smith PA, Romesberg FE. Combating bacteria and drug resistance by inhibiting mechanisms of persistence and adaptation. Nat Chem Biol. 2007;3:549–556. - PubMed
-
- Escaich S. Antivirulence as a new antibacterial approach for chemotherapy. Curr Opin Chem Biol. 2008;12:400–408. - PubMed
-
- Weidenmaier C, Kristian SA, Peschel A. Bacterial resistance to antimicrobial host defenses - an emerging target for novel antiinfective strategies? Curr Drug Targets. 2003;4:643–649. - PubMed
-
- Peschel A, Sahl HG. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol. 2006;4:529–536. - PubMed
-
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

