Two novel disaccharides, rutinose and methylrutinose, are involved in carbon metabolism in Datisca glomerata
- PMID: 19915863
- PMCID: PMC2806534
- DOI: 10.1007/s00425-009-1049-5
Two novel disaccharides, rutinose and methylrutinose, are involved in carbon metabolism in Datisca glomerata
Erratum in
- Planta. 2010 Feb;231(3):523
Abstract
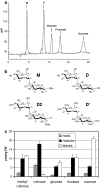
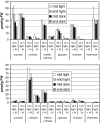
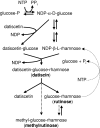
Datisca glomerata forms nitrogen-fixing root nodules in symbiosis with soil actinomycetes from the genus Frankia. Analysis of sugars in roots, nodules and leaves of D. glomerata revealed the presence of two novel compounds that were identified as alpha-L-rhamnopyranoside-(1 --> 6)-D-glucose (rutinose) and alpha-L-rhamnopyranoside-(1 --> 6)-1-O-beta-D-methylglucose (methylrutinose). Rutinose has been found previously as a/the glycoside part of several flavonoid glycosides, e.g. rutin, also of datiscin, the main flavonoid of Datisca cannabina, but had not been reported as free sugar. Time course analyses suggest that both rutinose and methylrutinose might play a role in transient carbon storage in sink organs and, to a lesser extent, in source leaves. Their concentrations show that they can accumulate in the vacuole. Rutinose, but not methylrutinose, was accepted as a substrate by the tonoplast disaccharide transporter SUT4 from Arabidopsis. In vivo (14)C-labeling and the study of uptake of exogenous sucrose and rutinose from the leaf apoplast showed that neither rutinose nor methylrutinose appreciably participate in phloem translocation of carbon from source to sink organs, despite rutinose being found in the apoplast at significant levels. A model for sugar metabolism in D. glomerata is presented.
Figures


References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:304–310. - PubMed
-
- Avigad G. Sucrose and other disaccharides. In: Loewus FA, Tanner W, editors. Plant carbohydrates I: intracellular carbohydrates. Encyclopedia of plant physiology, volume 13A. Berlin: Springer; 1982. pp. 217–347.
-
- Bar-Peled M, Lewinsohn E, Fluhr R, Gressel J. UDP-rhamnose:flavanone-7-O-glucoside-2′α″-O-rhamnosyltransferase. Purification and characterization of an enzyme catalyzing the production of bitter compounds in Citrus. J Biol Chem. 1991;226:20953–20959. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

