Cryptosporidium parvum induces B7-H1 expression in cholangiocytes by down-regulating microRNA-513
- PMID: 19916867
- PMCID: PMC2791176
- DOI: 10.1086/648589
Cryptosporidium parvum induces B7-H1 expression in cholangiocytes by down-regulating microRNA-513
Abstract
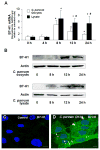
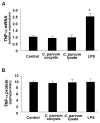
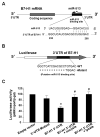
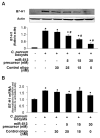
Expression of B7 costimulatory molecules represents an important compartment of immune response of epithelial cells after microbial infection. We report here that the protozoan parasite Cryptosporidium parvum induced B7-H1 expression in cultured human cholangiocytes. Induced expression of B7-H1 was identified in cells after exposure to infective C. parvum parasite or parasite lysate. Interestingly, the level of microRNA-513 (miR-513) was reduced in cells after exposure to C. parvum, which resulted in a relief of 3' untranslated region-mediated translational suppression of B7-H1. Overexpression of miR-513 through transfection of miR-513 precursor inhibited C. parvum-induced B7-H1 protein expression. Moreover, enhanced apoptotic cell death was identified in activated human T cells after coculture with C. parvum-infected cholangiocytes. The apoptosis of activated T cells was partially blocked by a neutralizing antibody to B7-H1 or transfection of cholangiocytes with miR-513 precursor. These data suggest a role of miR-513 in regulating B7-H1 expression by cholangiocytes in response to C. parvum infection.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures

Similar articles
-
MicroRNA-513 regulates B7-H1 translation and is involved in IFN-gamma-induced B7-H1 expression in cholangiocytes.J Immunol. 2009 Feb 1;182(3):1325-33. doi: 10.4049/jimmunol.182.3.1325. J Immunol. 2009. PMID: 19155478 Free PMC article.
-
The human immunodeficiency virus type 1 tat protein enhances Cryptosporidium parvum-induced apoptosis in cholangiocytes via a Fas ligand-dependent mechanism.Infect Immun. 2007 Feb;75(2):684-96. doi: 10.1128/IAI.01348-06. Epub 2006 Nov 21. Infect Immun. 2007. PMID: 17118988 Free PMC article.
-
MicroRNA-221 controls expression of intercellular adhesion molecule-1 in epithelial cells in response to Cryptosporidium parvum infection.Int J Parasitol. 2011 Mar;41(3-4):397-403. doi: 10.1016/j.ijpara.2010.11.011. Epub 2011 Jan 12. Int J Parasitol. 2011. PMID: 21236259 Free PMC article.
-
Significance of B7-H1 overexpression in kidney cancer.Clin Genitourin Cancer. 2006 Dec;5(3):206-11. doi: 10.3816/CGC.2006.n.038. Clin Genitourin Cancer. 2006. PMID: 17239274 Review.
-
Immunology of B7-H1 and its roles in human diseases.Int J Hematol. 2003 Nov;78(4):321-8. doi: 10.1007/BF02983556. Int J Hematol. 2003. PMID: 14686489 Review.
Cited by
-
The miRNA and mRNA Signatures of Peripheral Blood Cells in Humans Infected with Trypanosoma brucei gambiense.PLoS One. 2013 Jun 27;8(6):e67312. doi: 10.1371/journal.pone.0067312. Print 2013. PLoS One. 2013. PMID: 23826264 Free PMC article.
-
MiR-942-5p targeting the IFI27 gene regulates HCT-8 cell apoptosis via a TRAIL-dependent pathway during the early phase of Cryptosporidium parvum infection.Parasit Vectors. 2022 Aug 16;15(1):291. doi: 10.1186/s13071-022-05415-3. Parasit Vectors. 2022. PMID: 35974384 Free PMC article.
-
PD-L1-PD-1 Pathway in the Pathophysiology of Multiple Myeloma.Cancers (Basel). 2020 Apr 10;12(4):924. doi: 10.3390/cancers12040924. Cancers (Basel). 2020. PMID: 32290052 Free PMC article. Review.
-
microRNAs in parasites and parasite infection.RNA Biol. 2013 Mar;10(3):371-9. doi: 10.4161/rna.23716. Epub 2013 Feb 7. RNA Biol. 2013. PMID: 23392243 Free PMC article. Review.
-
Diagnostic Predictors of Immunotherapy Response in Head and Neck Squamous Cell Carcinoma.Diagnostics (Basel). 2023 Feb 23;13(5):862. doi: 10.3390/diagnostics13050862. Diagnostics (Basel). 2023. PMID: 36900006 Free PMC article. Review.
References
-
- Viswanathan VK, Hecht G. Innate immunity and the gut. Curr Opin Gastroenterol. 2000;16:546–51. - PubMed
-
- Han J, Ulevitch RJ. Limiting inflammatory responses during activation of innate immunity. Nat Immunol. 2005;6:1198–205. - PubMed
-
- Alpini G, McGill JM, LaRusso NF. The pathobiology of biliary epithelia. Hepatology. 2002;35:1256–68. - PubMed
-
- Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J Clin Gastroenterol. 2005;39:S90–102. - PubMed
-
- Chen XM, Keithly JS, Paya CV, LaRusso NF. Cryptosporidiosis. N Engl J Med. 2002;346:1723–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

