Inhaled tiotropium bromide and risk of stroke
- PMID: 19916997
- PMCID: PMC2791979
- DOI: 10.1111/j.1365-2125.2009.03517.x
Inhaled tiotropium bromide and risk of stroke
Abstract
Aims: A recent communication from the United States Food and Drug Administration highlighted a possible increased risk of stroke associated with use of the relatively new inhaled anticholinergic drug, tiotropium bromide. Using the United Kingdom General Practice Research Database, we set out to assess the risk of stroke in individuals exposed to inhaled tiotropium bromide and two other inhaled treatments for airways disease.
Methods: We used the self-controlled case-series that reduces confounding and minimizes the potential for biases in the quantification of risk estimates.
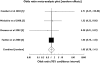
Results: Of 1043 people with a diagnosis of incident stroke who had had at least one prescription for tiotropium bromide, 980 satisfied inclusion criteria. The age-adjusted incidence rate ratio for all-cause stoke in individuals exposed to tiotropium bromide (n = 980), ipratropium bromide (n = 4181) and fluticasone propionate/salmeterol xinafoate (n = 1000) was 1.1 [95% confidence interval (CI) 0.9, 1.3], 0.8 (95% CI 0.7, 0.9) and 1.0 (95% CI 0.9, 1.2), respectively.
Conclusions: We found no evidence of an increased risk of all-cause stroke for individuals exposed to commonly prescribed inhaled treatments for chronic obstructive pulmonary disease.
Figures

 ); Drug Administration Period (
); Drug Administration Period ( ); Wash-out Period (
); Wash-out Period ( )
)
References
-
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–23. - PubMed
-
- US Food and Drug Administration. Early communication about an ongoing safety review of tiotropium (marketed as Spiriva HandiHaler) Available at http://www.fda.gov/cder/drug/early_comm/tiotropium.htm (last accessed 20 July 2008. - PubMed
-
- Spiriva HandiHaler (tiotropium bromide) Summary of Product Characteristics. Boehringer Ingelheim. Available at http://emc.medicines.org.uk/emc/industry/default.asp?page=displaydoc.asp... (last accessed 20 August 2008.
-
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–54. - PubMed
-
- Singh S, Loke YK, Furberg CD. Inhaled anticholinergics and risk of major adverse cardiovascular events in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA. 2008;300:1439–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

