Potentiation by WIN 55,212-2 of GABA-activated currents in rat trigeminal ganglion neurones
- PMID: 19917064
- PMCID: PMC2807652
- DOI: 10.1111/j.1476-5381.2009.00482.x
Potentiation by WIN 55,212-2 of GABA-activated currents in rat trigeminal ganglion neurones
Abstract
Background and purpose: Although both natural and synthetic cannabinoid compounds have been shown to exert an antinociceptive effect on acute and persistent pain, the anatomical locus of the target of cannabinoid-induced analgesia has not been fully elucidated. Here, we investigated the effects of the cannabinoid agonist WIN 55,212-2 on GABA-activated currents (I(GABA)) in rat primary sensory neurones.
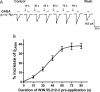
Experimental approach: In the present study, experiments were performed on neurones freshly isolated from rat trigeminal ganglion (TG) by using whole-cell patch clamp and repatch techniques.
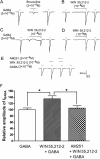
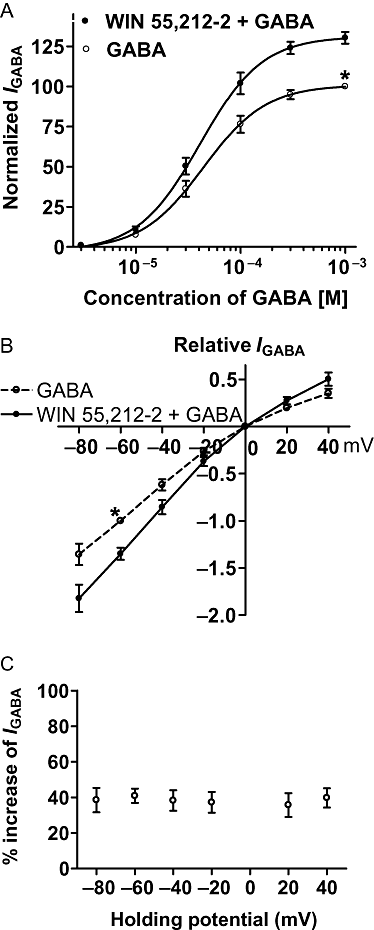
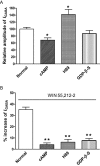
Key results: GABA-evoked inward currents were potentiated by pretreatment with WIN 55,212-2 in a concentration-dependent manner (10(-10)-10(-8) M). WIN 55,212-2 shifted the GABA concentration-response curve upwards, with an increase of 30.3 +/- 3.7% in the maximal current response but with no significant change in the EC(50) (agonist concentration producing a half-maximal response) value. WIN 55,212-2 potentiated the responses to GABA in a manner independent of holding potential and in the absence of any change in the reversal potential of the current. This potentiation of I(GABA) induced by WIN 55,212-2 was almost completely blocked by AM 251 (3 x 10(-8) M), a CB(1) receptor antagonist, and, using the repatch technique, was found to be abolished after intracellular dialysis with the protein kinase A (PKA) activator cAMP or the PKA inhibitor H89.
Conclusions and implications: The potentiation by WIN 55,212-2 of I(GABA) in primary sensory neurones may help to elucidate the mechanism underlying the modulation of analgesia by cannabinoids in the spinal dorsal horn.
Figures

References
-
- Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. - PubMed
-
- Bridges D, Rice AS, Egertová M, Elphick MR, Winter J, Michael GJ. Localisation of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridisation and immunohistochemistry. Neuroscience. 2003;119:803–812. - PubMed
-
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

