Factors necessary to produce basoapical polarity in human glandular epithelium formed in conventional and high-throughput three-dimensional culture: example of the breast epithelium
- PMID: 19917093
- PMCID: PMC2784453
- DOI: 10.1186/1741-7007-7-77
Factors necessary to produce basoapical polarity in human glandular epithelium formed in conventional and high-throughput three-dimensional culture: example of the breast epithelium
Abstract
Background: Basoapical polarity in epithelia is critical for proper tissue function, and control of proliferation and survival. Cell culture models that recapitulate epithelial tissue architecture are invaluable to unravel developmental and disease mechanisms. Although factors important for the establishment of basal polarity have been identified, requirements for the formation of apical polarity in three-dimensional tissue structures have not been thoroughly investigated.
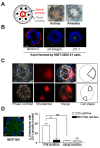
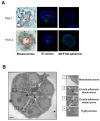
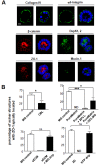
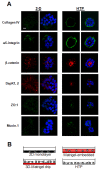
Results: We demonstrate that the human mammary epithelial cell line-3522 S1, provides a resilient model for studying the formation of basoapical polarity in glandular structures. Testing three-dimensional culture systems that differ in composition and origin of substrata reveals that apical polarity is more sensitive to culture conditions than basal polarity. Using a new high-throughput culture method that produces basoapical polarity in glandular structures without a gel coat, we show that basal polarity-mediated signaling and collagen IV are both necessary for the development of apical polarity.
Conclusion: These results provide new insights into the role of the basement membrane, and especially collagen IV, in the development of the apical pole, a critical element of the architecture of glandular epithelia. Also, the high-throughput culture method developed in this study should open new avenues for high-content screening of agents that act on mammary tissue homeostasis and thus, on architectural changes involved in cancer development.
Figures

Similar articles
-
Apical polarity in three-dimensional culture systems: where to now?J Biol. 2010;9(1):2. doi: 10.1186/jbiol213. Epub 2010 Jan 21. J Biol. 2010. PMID: 20092610 Free PMC article. Review.
-
Tissue polarity-dependent control of mammary epithelial homeostasis and cancer development: an epigenetic perspective.J Mammary Gland Biol Neoplasia. 2010 Mar;15(1):49-63. doi: 10.1007/s10911-010-9168-y. Epub 2010 Jan 27. J Mammary Gland Biol Neoplasia. 2010. PMID: 20101444 Free PMC article. Review.
-
The epithelial polarity axis controls the resting membrane potential and Cl- co-transport in breast glandular structures.J Cell Sci. 2024 Mar 1;137(5):jcs260924. doi: 10.1242/jcs.260924. Epub 2023 Nov 9. J Cell Sci. 2024. PMID: 37818620 Free PMC article.
-
The control of tissue architecture over nuclear organization is crucial for epithelial cell fate.J Cell Sci. 2007 May 1;120(Pt 9):1596-606. doi: 10.1242/jcs.03439. Epub 2007 Apr 3. J Cell Sci. 2007. PMID: 17405811
-
Cell polarity in motion: redefining mammary tissue organization through EMT and cell polarity transitions.J Mammary Gland Biol Neoplasia. 2010 Jun;15(2):149-68. doi: 10.1007/s10911-010-9180-2. Epub 2010 May 12. J Mammary Gland Biol Neoplasia. 2010. PMID: 20461450 Review.
Cited by
-
Development of an in vitro 3D tumor model to study therapeutic efficiency of an anticancer drug.Mol Pharm. 2013 Jun 3;10(6):2167-75. doi: 10.1021/mp300595a. Epub 2013 Mar 6. Mol Pharm. 2013. PMID: 23461341 Free PMC article.
-
Human eccrine sweat gland cells reconstitute polarized spheroids when subcutaneously implanted with Matrigel in nude mice.J Mol Histol. 2016 Oct;47(5):485-90. doi: 10.1007/s10735-016-9690-3. Epub 2016 Aug 4. J Mol Histol. 2016. PMID: 27492422
-
Peroxidasin Enhances Basal Phenotype and Inhibits Branching Morphogenesis in Breast Epithelial Progenitor Cell Line D492.J Mammary Gland Biol Neoplasia. 2021 Dec;26(4):321-338. doi: 10.1007/s10911-021-09507-1. Epub 2021 Dec 28. J Mammary Gland Biol Neoplasia. 2021. PMID: 34964086 Free PMC article.
-
3D Cultures of prostate cancer cells cultured in a novel high-throughput culture platform are more resistant to chemotherapeutics compared to cells cultured in monolayer.PLoS One. 2014 Nov 7;9(11):e111029. doi: 10.1371/journal.pone.0111029. eCollection 2014. PLoS One. 2014. PMID: 25380249 Free PMC article.
-
Modelling Cancer Pathophysiology: Mechanisms and Changes in the Extracellular Matrix During Cancer Initiation and Early Tumour Growth.Cancers (Basel). 2025 May 15;17(10):1675. doi: 10.3390/cancers17101675. Cancers (Basel). 2025. PMID: 40427172 Free PMC article. Review.
References
-
- Lelièvre SA, Bissell MJ. In: Encyclopedia of Molecular Cell Biology and Molecular Medicine. 2. Meyers RA, editor. Vol. 14. Weinheim: Wiley-VCH; 2005. Three-dimensional cell culture: The importance of context in regulation of function; pp. 383–420.
-
- Jaeger MM, Dodane V, Kachar B. Modulation of tight junction morphology and permeability by an epithelial factor. J Membr Biol. 1994;139(1):41–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

