High-chloride concentrations abolish the binding of adenine nucleotides in the mitochondrial ADP/ATP carrier family
- PMID: 19917217
- PMCID: PMC2776247
- DOI: 10.1016/j.bpj.2009.08.047
High-chloride concentrations abolish the binding of adenine nucleotides in the mitochondrial ADP/ATP carrier family
Abstract
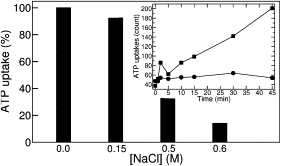
The ADP/ATP carrier (AAC) is a very effective membrane protein that mediates the exchange of ADP and ATP across the mitochondrial membrane. In vivo transport measurements on the AAC overexpressed in Escherichia coli demonstrate that this process can be severely inhibited by high-chloride concentrations. Molecular-dynamics simulations reveal a strong modification of the topology of the local electric field related to the number of chloride ions inside the cavity. Halide ions are shown to shield the positive charges lining the internal cavity of the carrier by accurate targeting of key basic residues. These specific amino acids are highly conserved as highlighted by the analysis of multiple AAC sequences. These results strongly suggest that the chloride concentration acts as an electrostatic lock for the mitochondrial AAC family, thereby preventing adenine nucleotides from reaching their dedicated binding sites.
Figures


References
-
- Dahout-Gonzalez C., Nury H., Trézéguet V., Lauquin G.J.-M., Pebay-Peyroula E. Molecular, functional, and pathological aspects of the mitochondrial ADP/ATP carrier. Physiology (Bethesda) 2006;21:242–249. - PubMed
-
- Reference deleted in proof.
-
- Reference deleted in proof.
-
- Reference deleted in proof.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

