Kinetics of the multistep rupture of fibrin 'A-a' polymerization interactions measured using atomic force microscopy
- PMID: 19917237
- PMCID: PMC2776257
- DOI: 10.1016/j.bpj.2009.08.042
Kinetics of the multistep rupture of fibrin 'A-a' polymerization interactions measured using atomic force microscopy
Abstract
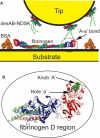
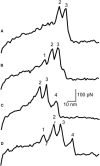
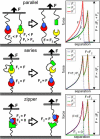
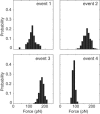
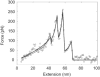
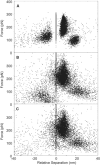
Fibrin, the structural scaffold of blood clots, spontaneously polymerizes through the formation of 'A-a' knob-hole bonds. When subjected to external force, the dissociation of this bond is accompanied by two to four abrupt changes in molecular dimension observable as rupture events in a force curve. Herein, the configuration, molecular extension, and kinetic parameters of each rupture event are examined. The increases in contour length indicate that the D region of fibrinogen can lengthen by approximately 50% of the length of a fibrin monomer before rupture of the 'A-a' interaction. The dependence of the dissociation rate on applied force was obtained using probability distributions of rupture forces collected at different pull-off velocities. These distributions were fit using a model in which the effects of the shape of the binding potential are used to quantify the kinetic parameters of forced dissociation. We found that the weak initial rupture (i.e., event 1) was not well approximated by these models. The ruptured bonds comprising the strongest ruptures, events 2 and 3, had kinetic parameters similar to those commonly found for the mechanical unfolding of globular proteins. The bonds ruptured in event 4 were well described by these analyses, but were more loosely bound than the bonds in events 2 and 3. We propose that the first event represents the rupture of an unknown interaction parallel to the 'A-a' bond, events 2 and 3 represent unfolding of structures in the D region of fibrinogen, and event 4 is the rupture of the 'A-a' knob-hole bond weakened by prior structural unfolding. Comparison of the activation energy obtained via force spectroscopy measurements with the thermodynamic free energy of 'A-a' bond dissociation indicates that the 'A-a' bond may be more resistant to rupture by applied force than to rupture by thermal dissociation.
Figures

Similar articles
-
Molecular mechanisms, thermodynamics, and dissociation kinetics of knob-hole interactions in fibrin.J Biol Chem. 2013 Aug 2;288(31):22681-92. doi: 10.1074/jbc.M113.472365. Epub 2013 May 28. J Biol Chem. 2013. PMID: 23720752 Free PMC article.
-
Calcium dependence of fibrin nanomechanics: the γ1 calcium mediates the unfolding of fibrinogen induced by force applied to the "A-a" bond.Langmuir. 2010 Sep 21;26(18):14716-22. doi: 10.1021/la1017664. Langmuir. 2010. PMID: 20731339
-
Recombinant γT305A fibrinogen indicates severely impaired fibrin polymerization due to the aberrant function of hole 'A' and calcium binding sites.Thromb Res. 2014 Aug;134(2):518-25. doi: 10.1016/j.thromres.2014.06.002. Epub 2014 Jun 11. Thromb Res. 2014. PMID: 24968960
-
Practical single molecule force spectroscopy: how to determine fundamental thermodynamic parameters of intermolecular bonds with an atomic force microscope.Methods. 2013 Apr 1;60(2):142-50. doi: 10.1016/j.ymeth.2013.03.014. Epub 2013 Mar 24. Methods. 2013. PMID: 23531626 Review.
-
Probing the relation between force--lifetime--and chemistry in single molecular bonds.Annu Rev Biophys Biomol Struct. 2001;30:105-28. doi: 10.1146/annurev.biophys.30.1.105. Annu Rev Biophys Biomol Struct. 2001. PMID: 11340054 Review.
Cited by
-
Microscale structural changes of individual fibrin fibers during fibrinolysis.Acta Biomater. 2022 Mar 15;141:114-122. doi: 10.1016/j.actbio.2022.01.006. Epub 2022 Jan 7. Acta Biomater. 2022. PMID: 35007782 Free PMC article.
-
Structural Mechanisms of Forced Unfolding of Double-Stranded Fibrin Oligomers.J Phys Chem B. 2025 Apr 24;129(16):3963-3977. doi: 10.1021/acs.jpcb.5c00755. Epub 2025 Apr 14. J Phys Chem B. 2025. PMID: 40227118 Free PMC article.
-
Engineered Molecular Therapeutics Targeting Fibrin and the Coagulation System: a Biophysical Perspective.Biophys Rev. 2022 Apr 6;14(2):427-461. doi: 10.1007/s12551-022-00950-w. eCollection 2022 Apr. Biophys Rev. 2022. PMID: 35399372 Free PMC article. Review.
-
Fibrin mechanical properties and their structural origins.Matrix Biol. 2017 Jul;60-61:110-123. doi: 10.1016/j.matbio.2016.08.003. Epub 2016 Aug 20. Matrix Biol. 2017. PMID: 27553509 Free PMC article. Review.
-
Integration of acoustic radiation force and optical imaging for blood plasma clot stiffness measurement.PLoS One. 2015 Jun 4;10(6):e0128799. doi: 10.1371/journal.pone.0128799. eCollection 2015. PLoS One. 2015. PMID: 26042775 Free PMC article.
References
-
- Laurens N., Koolwijk P., de Maat M.P. Fibrin structure and wound healing. J. Thromb. Haemost. 2006;4:932–939. - PubMed
-
- Weisel J.W. Fibrinogen and fibrin. Adv. Protein Chem. 2005;70:247–299. - PubMed
-
- Weisel J. Which knobs fit into which holes in fibrin polymerization? J. Thromb. Haemost. 2007;5:2340–2343. - PubMed
-
- Weisel J.W. The mechanical properties of fibrin for basic scientists and clinicians. Biophys. Chem. 2004;112:267–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

