The unique kinetics of iron release from transferrin: the role of receptor, lobe-lobe interactions, and salt at endosomal pH
- PMID: 19917294
- PMCID: PMC2815179
- DOI: 10.1016/j.jmb.2009.11.023
The unique kinetics of iron release from transferrin: the role of receptor, lobe-lobe interactions, and salt at endosomal pH
Abstract
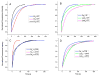
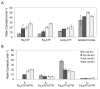
Transferrins are a family of bilobal iron-binding proteins that play the crucial role of binding ferric iron and keeping it in solution, thereby controlling the levels of this important metal. Human serum transferrin (hTF) carries one iron in each of two similar lobes. Understanding the detailed mechanism of iron release from each lobe of hTF during receptor-mediated endocytosis has been extremely challenging because of the active participation of the transferrin receptor (TFR), salt, a chelator, lobe-lobe interactions, and the low pH within the endosome. Our use of authentic monoferric hTF (unable to bind iron in one lobe) or diferric hTF (with iron locked in one lobe) provided distinct kinetic end points, allowing us to bypass many of the previous difficulties. The capture and unambiguous assignment of all kinetic events associated with iron release by stopped-flow spectrofluorimetry, in the presence and in the absence of the TFR, unequivocally establish the decisive role of the TFR in promoting efficient and balanced iron release from both lobes of hTF during one endocytic cycle. For the first time, the four microscopic rate constants required to accurately describe the kinetics of iron removal are reported for hTF with and without the TFR. Specifically, at pH 5.6, the TFR enhances the rate of iron release from the C-lobe (7-fold to 11-fold) and slows the rate of iron release from the N-lobe (6-fold to 15-fold), making them more equivalent and producing an increase in the net rate of iron removal from Fe(2)hTF. Calculated cooperativity factors, in addition to plots of time-dependent species distributions in the absence and in the presence of the TFR, clearly illustrate the differences. Accurate rate constants for the pH and salt-induced conformational changes in each lobe precisely delineate how delivery of iron within the physiologically relevant time frame of 2 min might be accomplished.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Kinetics of iron release from transferrin bound to the transferrin receptor at endosomal pH.Biochim Biophys Acta. 2012 Mar;1820(3):326-33. doi: 10.1016/j.bbagen.2011.06.003. Epub 2011 Jun 15. Biochim Biophys Acta. 2012. PMID: 21699959 Free PMC article. Review.
-
Ionic residues of human serum transferrin affect binding to the transferrin receptor and iron release.Biochemistry. 2012 Jan 17;51(2):686-94. doi: 10.1021/bi201661g. Epub 2012 Jan 6. Biochemistry. 2012. PMID: 22191507 Free PMC article.
-
How the binding of human transferrin primes the transferrin receptor potentiating iron release at endosomal pH.Proc Natl Acad Sci U S A. 2011 Aug 9;108(32):13089-94. doi: 10.1073/pnas.1105786108. Epub 2011 Jul 25. Proc Natl Acad Sci U S A. 2011. PMID: 21788477 Free PMC article.
-
Evidence that His349 acts as a pH-inducible switch to accelerate receptor-mediated iron release from the C-lobe of human transferrin.J Biol Inorg Chem. 2010 Nov;15(8):1341-52. doi: 10.1007/s00775-010-0694-2. Epub 2010 Aug 14. J Biol Inorg Chem. 2010. PMID: 20711621 Free PMC article.
-
Transferrin-mediated cellular iron delivery.Curr Top Membr. 2012;69:3-35. doi: 10.1016/B978-0-12-394390-3.00001-X. Curr Top Membr. 2012. PMID: 23046645 Free PMC article. Review.
Cited by
-
Structure and dynamics of drug carriers and their interaction with cellular receptors: focus on serum transferrin.Adv Drug Deliv Rev. 2013 Jul;65(8):1012-9. doi: 10.1016/j.addr.2012.11.001. Epub 2012 Nov 23. Adv Drug Deliv Rev. 2013. PMID: 23183585 Free PMC article. Review.
-
Desferrithiocin: a search for clinically effective iron chelators.J Med Chem. 2014 Nov 26;57(22):9259-91. doi: 10.1021/jm500828f. Epub 2014 Sep 10. J Med Chem. 2014. PMID: 25207964 Free PMC article. Review.
-
Kinetics of iron release from transferrin bound to the transferrin receptor at endosomal pH.Biochim Biophys Acta. 2012 Mar;1820(3):326-33. doi: 10.1016/j.bbagen.2011.06.003. Epub 2011 Jun 15. Biochim Biophys Acta. 2012. PMID: 21699959 Free PMC article. Review.
-
Ionic residues of human serum transferrin affect binding to the transferrin receptor and iron release.Biochemistry. 2012 Jan 17;51(2):686-94. doi: 10.1021/bi201661g. Epub 2012 Jan 6. Biochemistry. 2012. PMID: 22191507 Free PMC article.
-
The long history of iron in the Universe and in health and disease.Biochim Biophys Acta. 2012 Mar;1820(3):161-87. doi: 10.1016/j.bbagen.2011.08.002. Epub 2011 Aug 9. Biochim Biophys Acta. 2012. PMID: 21856378 Free PMC article. Review.
References
-
- Hall DR, Hadden JM, Leonard GA, Bailey S, Neu M, Winn M, Lindley PF. The crystal and molecular structures of diferric porcine and rabbit serum transferrins at resolutions of 2.15 and 2.60 Å, respectively. Acta Crystallogr D Biol Crystallogr. 2002;58:70–80. - PubMed
-
- Mason AB, Halbrooks PJ, James NG, Connolly SA, Larouche JR, Smith VC, MacGillivray RT, Chasteen ND. Mutational analysis of C-lobe ligands of human serum transferrin: insights into the mechanism of iron release. Biochemistry. 2005;44:8013–21. - PubMed
-
- Klausner RD, Van Renswoude J, Ashwell G, Kempf C, Schechter AN, Dean A, Bridges KR. Receptor-mediated endocytosis of transferrin in K562 cells. J Biol Chem. 1983;258:4715–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

