Specific real-time reverse transcription-polymerase chain reaction for detection and quantitation of turkey coronavirus RNA in tissues and feces from turkeys infected with turkey coronavirus
- PMID: 19917315
- PMCID: PMC7112835
- DOI: 10.1016/j.jviromet.2009.11.012
Specific real-time reverse transcription-polymerase chain reaction for detection and quantitation of turkey coronavirus RNA in tissues and feces from turkeys infected with turkey coronavirus
Abstract
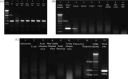
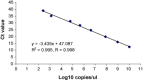
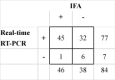
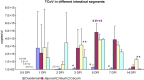
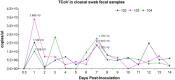
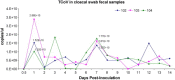
Turkey coronavirus (TCoV) infection causes acute atrophic enteritis in the turkey poults, leading to significant economic loss in the U.S. turkey industry. Rapid detection, differentiation, and quantitation of TCoV are critical to the diagnosis and control of the disease. A specific one-step real-time reverse transcription-polymerase chain reaction (RRT-PCR) assay for detection and quantitation of TCoV in the turkey tissues was developed using a dual-labeled fluorescent probe. The fluorogenic probe labeled with a reporter dye (FAM, 6-carboxytetramethylrhodamin) and a quencher dye (AbsoluteQuencher) was designed to bind to a 186 base-pair fragment flanked by the two PCR primers targeting the 3' end of spike gene of TCoV. The assay was performed on different avian viruses and bacteria to determine the specificity as well as serial dilutions of TCoV for the sensitivity. Three animal trials were conducted to further validate the assay. Ten-day-old turkey poults were inoculated orally with 100 EID(50) of TCoV. Intestinal tissues (duodenum, jejunum, ileum, cecum), feces from the cloacal swabs, or feces from the floor were collected at 12 h, 1, 2, 3, 5, 7, and/or 14 days post-inoculation (DPI). RNA was extracted from each sample and subjected to the RRT-PCR. The designed primers and probe were specific for TCoV. Other non-TCoV avian viruses and bacteria were not amplified by RRT-PCR. The assay was highly sensitive and could quantitate between 10(2) and 10(10) copies/microl of viral genome. The viral RNA in the intestine segments reached the highest level, 6x10(15) copies/microl, in the jejunum at 5 DPI. Eighty-four intestine segments assayed by the developed RRT-PCR and immunofluorescence antibody assay (IFA) revealed that there were 6 segments negative for TCoV by both assays, 45 positive for TCoV by IFA, and 77 positive for TCoV by RRT-PCR. Turkey coronavirus was detected in the feces from the cloacal swabs or floor 1-14 DPI; however, the viral RNA load varied among different turkey poults at different intervals from different trials. The highest amount of viral RNA, 2.8x10(10) copies/microl, in the feces was the one from the cloacal swab collected at 1 DPI. The average amount of TCoV RNA in the cloacal fecal samples was 10 times higher than that in the fecal droppings on the floor. Taken together, the results indicated that the developed RRT-PCR assay is rapid, sensitive, and specific for detection, differentiation, and quantitation of TCoV in the turkey tissues and should be helpful in monitoring the progression of TCoV induced acute enteritis in the turkey flocks.
2009 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
A DNA prime-protein boost vaccination strategy targeting turkey coronavirus spike protein fragment containing neutralizing epitope against infectious challenge.Vet Immunol Immunopathol. 2013 Apr 15;152(3-4):359-69. doi: 10.1016/j.vetimm.2013.01.009. Epub 2013 Feb 1. Vet Immunol Immunopathol. 2013. PMID: 23428360 Free PMC article.
-
First full-length sequences of the S gene of European isolates reveal further diversity among turkey coronaviruses.Avian Pathol. 2011 Apr;40(2):179-89. doi: 10.1080/03079457.2011.551936. Avian Pathol. 2011. PMID: 21500038
-
Pathology and tissue distribution of turkey coronavirus in experimentally infected chicks and turkey poults.J Comp Pathol. 2010 Jul;143(1):8-13. doi: 10.1016/j.jcpa.2009.12.012. Epub 2010 Feb 16. J Comp Pathol. 2010. PMID: 20163804 Free PMC article.
-
Effect of coronavirus infection on reproductive performance of turkey hens.Avian Dis. 2013 Sep;57(3):650-6. doi: 10.1637/10502-012513-Reg.1. Avian Dis. 2013. PMID: 24283132
-
Detection of turkey coronavirus in commercial turkey poults in Brazil.Avian Pathol. 2007 Feb;36(1):29-33. doi: 10.1080/03079450601102939. Avian Pathol. 2007. PMID: 17364507
Cited by
-
Enteric viruses in turkey enteritis.Virusdisease. 2014;25(2):173-85. doi: 10.1007/s13337-014-0198-8. Epub 2014 Feb 19. Virusdisease. 2014. PMID: 25674583 Free PMC article.
-
Coronavirus in Recent Years: Characteristics, Outbreaks, and Treatments.Tanaffos. 2024 Mar;23(3):220-237. Tanaffos. 2024. PMID: 40704343 Free PMC article. Review.
-
Upregulation of INF-γ, IL-6, and IL-8 expression during replication of turkey coronavirus in nonepithelial cells obtained from Meleagris gallopavo.Arch Virol. 2021 Aug;166(8):2285-2289. doi: 10.1007/s00705-021-05120-z. Epub 2021 May 31. Arch Virol. 2021. Retraction in: Arch Virol. 2022 Dec;167(12):2925. doi: 10.1007/s00705-022-05620-6. PMID: 34057608 Free PMC article. Retracted.
-
A DNA prime-protein boost vaccination strategy targeting turkey coronavirus spike protein fragment containing neutralizing epitope against infectious challenge.Vet Immunol Immunopathol. 2013 Apr 15;152(3-4):359-69. doi: 10.1016/j.vetimm.2013.01.009. Epub 2013 Feb 1. Vet Immunol Immunopathol. 2013. PMID: 23428360 Free PMC article.
-
Visual detection of turkey coronavirus RNA in tissues and feces by reverse-transcription loop-mediated isothermal amplification (RT-LAMP) with hydroxynaphthol blue dye.Mol Cell Probes. 2010 Dec;24(6):415-7. doi: 10.1016/j.mcp.2010.08.003. Epub 2010 Aug 21. Mol Cell Probes. 2010. PMID: 20732411 Free PMC article.
References
-
- Akin A., Lin T.L., Wu C.C., Bryan T.A., Hooper T., Schrader D. Nucleocapsid protein gene sequence analysis reveals close genomic relationship between turkey coronavirus and avian infectious bronchitis virus. Acta Virol. 2001;45:31–38. - PubMed
-
- Breslin J.J., Smith L.G., Barnes H.J., Guy J.S. Comparison of virus isolation, immunohistochemistry, and reverse transcriptase-polymerase chain reaction procedures for detection of turkey coronavirus. Avian Dis. 2000;44:624–631. - PubMed
-
- Cavanagh D. Coronaviruses in poultry and other birds. Avian Pathol. 2005;34:439–448. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

