Cowpox virus inhibits the transporter associated with antigen processing to evade T cell recognition
- PMID: 19917498
- PMCID: PMC2791678
- DOI: 10.1016/j.chom.2009.09.013
Cowpox virus inhibits the transporter associated with antigen processing to evade T cell recognition
Abstract
Cowpox virus encodes an extensive array of putative immunomodulatory proteins, likely contributing to its wide host range, which includes zoonotic infections in humans. Unlike Vaccinia virus, cowpox virus prevents stimulation of CD8(+) T cells, a block that correlated with retention of MHC class I in the endoplasmic reticulum by the cowpox virus protein CPXV203. However, deletion of CPXV203 did not restore MHC class I transport or T cell stimulation. Here, we demonstrate the contribution of an additional viral protein, CPXV12, which interferes with MHC class I/peptide complex formation by inhibiting peptide translocation by the transporter associated with antigen processing (TAP). Importantly, human and mouse MHC class I transport and T cell stimulation was restored upon deletion of both CPXV12 and CPXV203, suggesting that these unrelated proteins independently mediate T cell evasion in multiple hosts. CPXV12 is a truncated version of a putative NK cell ligand, indicating that poxviral gene fragments can encode new, unexpected functions.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

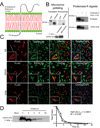
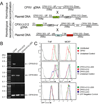

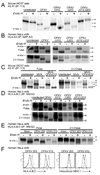
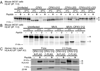
Comment in
-
Jenner's irony: cowpox taps into T cell evasion.Cell Host Microbe. 2009 Nov 19;6(5):395-7. doi: 10.1016/j.chom.2009.11.001. Cell Host Microbe. 2009. PMID: 19917492
Similar articles
-
Two mechanistically distinct immune evasion proteins of cowpox virus combine to avoid antiviral CD8 T cells.Cell Host Microbe. 2009 Nov 19;6(5):422-32. doi: 10.1016/j.chom.2009.09.012. Cell Host Microbe. 2009. PMID: 19917497 Free PMC article.
-
Cowpox virus exploits the endoplasmic reticulum retention pathway to inhibit MHC class I transport to the cell surface.Cell Host Microbe. 2007 Nov 15;2(5):306-15. doi: 10.1016/j.chom.2007.09.002. Cell Host Microbe. 2007. PMID: 18005752
-
Cowpox virus protein CPXV012 eludes CTLs by blocking ATP binding to TAP.J Immunol. 2014 Aug 15;193(4):1578-89. doi: 10.4049/jimmunol.1400964. Epub 2014 Jul 14. J Immunol. 2014. PMID: 25024387 Free PMC article.
-
Cowpox virus employs a two-pronged strategy to outflank MHCI antigen presentation.Mol Immunol. 2013 Sep;55(2):156-8. doi: 10.1016/j.molimm.2012.11.011. Epub 2013 Jan 10. Mol Immunol. 2013. PMID: 23312338 Free PMC article. Review.
-
Modulation of the host immune response by cowpox virus.Microbes Infect. 2010 Nov;12(12-13):900-9. doi: 10.1016/j.micinf.2010.07.007. Epub 2010 Jul 29. Microbes Infect. 2010. PMID: 20673807 Free PMC article. Review.
Cited by
-
Orf virus interferes with MHC class I surface expression by targeting vesicular transport and Golgi.BMC Vet Res. 2012 Jul 18;8:114. doi: 10.1186/1746-6148-8-114. BMC Vet Res. 2012. PMID: 22809544 Free PMC article.
-
ABC proteins in antigen translocation and viral inhibition.Nat Chem Biol. 2010 Aug;6(8):572-80. doi: 10.1038/nchembio.410. Nat Chem Biol. 2010. PMID: 20644544 Review.
-
Know Thyself: NK-Cell Inhibitory Receptors Prompt Self-Tolerance, Education, and Viral Control.Front Immunol. 2014 Apr 16;5:175. doi: 10.3389/fimmu.2014.00175. eCollection 2014. Front Immunol. 2014. PMID: 24795719 Free PMC article. Review.
-
Characterization of a large, proteolytically processed cowpox virus membrane glycoprotein conserved in most chordopoxviruses.Virology. 2015 Sep;483:209-17. doi: 10.1016/j.virol.2015.04.014. Epub 2015 May 15. Virology. 2015. PMID: 25980741 Free PMC article.
-
Antiviral immunity following smallpox virus infection: a case-control study.J Virol. 2010 Dec;84(24):12754-60. doi: 10.1128/JVI.01763-10. Epub 2010 Oct 6. J Virol. 2010. PMID: 20926574 Free PMC article.
References
-
- Bacik I, Cox JH, Anderson R, Yewdell JW, Bennink JR. TAP (transporter associated with antigen processing)-independent presentation of endogenously synthesized peptides is enhanced by endoplasmic reticulum insertion sequences located at the amino- but not carboxyl-terminus of the peptide. J Immunol. 1994;152:381–387. - PubMed
-
- Bakker AH, Hoppes R, Linnemann C, Toebes M, Rodenko B, Berkers CR, Hadrup SR, van Esch WJ, Heemskerk MH, Ovaa H, et al. Conditional MHC class I ligands and peptide exchange technology for the human MHC gene products HLA-A1, -A3, - A11, and -B7. Proc Natl Acad Sci U S A. 2008;105:3825–3830. - PMC - PubMed
-
- Banks TA, Jenkins FJ, Kanangat S, Nair S, Dasgupta S, Foster CM, Rouse BT. Vaccination with the immediate-early protein ICP47 of herpes simplex virus-type 1 (HSV-1) induces virus-specific lymphoproliferation, but fails to protect against lethal challenge. Virology. 1994;200:236–245. - PubMed
-
- Barel MT, Pizzato N, Le Bouteiller P, Wiertz EJ, Lenfant F. Subtle sequence variation among MHC class I locus products greatly influences sensitivity to HCMV US2- and US11-mediated degradation. International immunology. 2006;18:173–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

