Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection
- PMID: 19917501
- PMCID: PMC2785497
- DOI: 10.1016/j.chom.2009.10.007
Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection
Abstract
Aspergillus fumigatus, a ubiquitous fungus, causes invasive disease in immunocompromised humans. Although monocytes and antigen-specific CD4 T cells contribute to defense against inhaled fungal spores, how these cells interact during infection remains undefined. Investigating the role of inflammatory monocytes and monocyte-derived dendritic cells during fungal infection, we find that A. fumigatus infection induces an influx of chemokine receptor CCR2- and Ly6C-expressing inflammatory monocytes into lungs and draining lymph nodes. Depletion of CCR2(+) cells reduced A. fumigatus conidial transport from lungs to draining lymph nodes, abolished CD4 T cell priming following respiratory challenge, and impaired pulmonary fungal clearance. In contrast, depletion of CCR2(+)Ly6C(hi) monocytes during systemic fungal infection did not prevent CD4 T cell priming in the spleen. Our findings demonstrate that pulmonary CD4 T cell responses to inhaled spores require CCR2(+)Ly6C(hi) monocytes and their derivatives, revealing a compartmentally restricted function for these cells in adaptive respiratory immune responses.
Figures
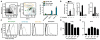
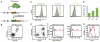
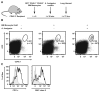
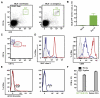


References
-
- Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009b;27:669–692. - PubMed
-
- Beck O, Topp MS, Koehl U, Roilides E, Simitsopoulou M, Hanisch M, Sarfati J, Latge JP, Klingebiel T, Einsele H, Lehrnbecher T. Generation of highly purified and functionally active human TH1 cells against Aspergillus fumigatus. Blood. 2006;107:2562–2569. - PubMed
-
- Belz GT, Smith CM, Kleinert L, Reading P, Brooks A, Shortman K, Carbone FR, Heath WR. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc. Natl. Acad. Sci. USA. 2004;101:8670–8675. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

