Gating of nicotinic ACh receptors: latest insights into ligand binding and function
- PMID: 19917567
- PMCID: PMC2828134
- DOI: 10.1113/jphysiol.2009.182691
Gating of nicotinic ACh receptors: latest insights into ligand binding and function
Abstract
Nicotinic acetylcholine receptors (nAChRs) are in the superfamily of cys-loop receptors, and are widely expressed in the nervous system where they participate in a variety of physiological functions, including regulating excitability and neurotransmitter release, as well as neuromuscular contraction. Members of the cys-loop family of receptors, which also includes the molluscan ACh-binding protein (AChBP), a soluble protein that is analogous to the extracellular ligand-binding domain of the cys-loop receptors, are pentameric assemblies of five subunits, with each subunit arranged around a central pore. The binding of ACh to the extracellular interface between two subunits induces channel opening. With the recent 4 A resolution of the Torpedo nAChR, and the crystal structure of the AChBP, much has been learned about the structure of the ligand-binding domain and the channel pore, as well as major structural rearrangements that may confer channel opening, including a major rearrangement of the C-loop within the ligand binding pocket, and perhaps other regions including the F-loop (the beta8-beta9 linker), the beta1-beta2 linker and the cys-loop. Here I will review the latest findings from my lab aimed at a further understanding of the function of the neuronal nAChR channels (and in particular the role of desensitization), and our search for novel AChBP species that may lead to a further understanding of the function of the cys-loop receptor family.
Figures

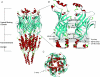
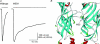
References
-
- Bertrand D, Gopalakrishnan M. Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74:1155–1163. - PubMed
-
- Bouzat C, Gumilar F, Spitzmaul G, Wang HL, Rayes D, Hansen SB, Taylor P, Sine SM. Coupling of agonist binding to channel gating in an ACh-binding protein linked to an ion channel. Nature. 2004;430:896–900. - PubMed
-
- Brejc K, Van Dijk WJ, Klaassen RV, Schuurmans M, Van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

